
A new study suggests that individuals taking semaglutide, the active ingredient in drugs like Ozempic and Wegovy, may be at a higher risk for developing a rare blinding condition known as Nonarteritic Anterior Ischemic Optic Neuropathy (NAION). Individuals taking the drug have reported vision loss, blurry vision, and even blindness, raising concerns over the safety of the drug. Litigation continues alleging the manufacturer of Wegovy and Ozempic knew the risk of harm to patients and failed to warn consumers. It is expected that more lawsuits will be filed with the widespread use of these drugs.
Wegovy Vision Loss Lawsuit Overview
According to legal documents, patients allege that Novo Nordisk, the manufacturer of Wegovy, knew the drug might increase a person’s risk of developing serious eye injuries such as Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) and failed to warn consumers. Original labels on the prescription drug did not contain warnings about the potential for vision problems, including sudden blindness or severe gastrointestinal issues.
Individuals who have taken Wegovy and experienced serious eye injuries, such as blurry vision or sudden blindness, are encouraged to contact King Law to schedule a free consultation. Depending on the circumstances, aggrieved consumers may be entitled to take legal action. Novo Nordisk continues to face legal and financial repercussions as concerns grow over whether the drug causes serious, life-changing side effects.
Wegovy and Vision Problems – 2025 Update
July 2024: A new study, published on July 3, 2024, indicates patients taking semaglutide such as Wegovy may face an increased risk of serious eye injuries, including Nonarteritic Anterior Ischemic Optic Neuropathy (NAION). NAION can be debilitating and may result in vision loss. The study followed more than 16,000 patients over the course of six years and found a higher incidence of the condition. Individuals with diabetes taking semaglutide may be at a 4x greater risk for developing NAION.
About the Wegovy Vision Loss Lawsuit:
What Is Vision Loss From Wegovy?
How Does Wegovy Cause Vision Problems?
Wegovy Vision Issues and Eye Side Effects
New JAMA Study Links Wegovy to NAION
Novo Nordisk’s Response to JAMA Study and Allegations
Percentage of Wegovy Users Reporting Vision Problems
How to File a Wegovy Vision Loss Lawsuit
What Is Vision Loss From Wegovy?
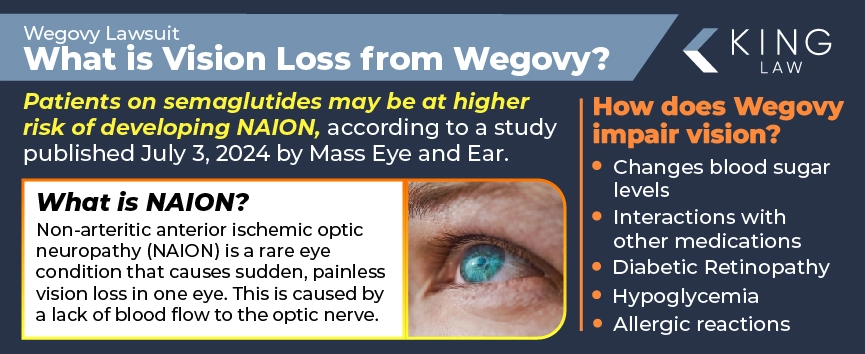
On July 3, 2024, a concerning study published by researchers from Mass Eye and Ear found that patients taking semaglutide, the active ingredient in drugs like Ozempic and Wegovy, may be at a higher risk of developing a rare blinding condition known as Nonarteritic Anterior Ischemic Optic Neuropathy (NAION).
NAION is marked by decreased blood supply to the optic nerve and can lead to sudden blindness. It is the second most common form of optic nerve damage and is frequently referred to as a “stroke of the optic nerve.” The optic nerve is a bundle of fibers that connects the eye to the brain, enabling vision. As the blood flow to the optic nerve is restricted, vision is impaired. The condition is rare, with an annual incidence rate estimated between 2.3 to 10.2 per 100,000 people over 50 years of age in the U.S.
Individuals taking Wegovy, particularly older adults, may be at a higher risk of developing NAION and should seek medical attention immediately for blurred vision, blindness, or other sight concerns. The debilitating eye condition may result in permanent vision loss.
How Does Wegovy Cause Vision Problems?
Research and legal documents allege that semaglutide, the active ingredient in the popular weight loss drug Wegovy, may cause blindness and vision problems. It is strongly recommended that patients taking the prescription medication who experience disturbances in their vision seek medical attention immediately.
Ways Wegovy May Affect Vision:
- Fluctuating Blood Sugar Levels: Wegovy helps regulate blood sugar levels. Significant fluctuations in blood sugar levels can damage blood vessels in the retina if not well-controlled, potentially leading to serious vision problems and even blindness if left untreated.
- Diabetic Retinopathy: Patients with diabetes are at an increased risk for developing diabetic retinopathy due to uncontrolled blood sugar levels. High blood sugar can cause a blockage of blood vessels, cutting off blood supply to the retina. While Wegovy is not directly linked to the condition, its role in managing diabetes may impact the risk.
- Hypoglycemia (Low Blood Sugar): Wegovy may cause hypoglycemia, a condition defined by low blood sugar. Hypoglycemia can result in blurred vision or tunnel vision, but the symptoms generally resolve once blood sugar levels normalize.
- Side Effects of Other Medications: Many patients taking Wegovy for diabetes management may also be taking other medications to help manage the condition. These medications, in combination with Wegovy, may increase the risk of developing vision problems.
- Allergic Reactions or Adverse Effects: In rare cases, some patients taking Wegovy
Any unusual symptoms that come up while taking Wegovy should be reported to a healthcare provider immediately. Regular eye exams and monitoring of blood sugar levels can help manage and prevent vision-related issues.
Wegovy Vision Issues and Eye Side Effects
A new study published by researchers at Mass Eye and Ear indicates that patients taking Wegovy may be at an increased risk for developing severe eye problems, including Non-arteritic Anterior Ischemic Optic Neuropathy (NAION). NAION can result in the sudden, painless loss of vision in one eye. In addition to NAION, Wegovy patients may also face temporary blurred vision from fluctuating blood sugar levels, an increase in eye infections, and other vision issues.
Wegovy vision issues and potential side effects:
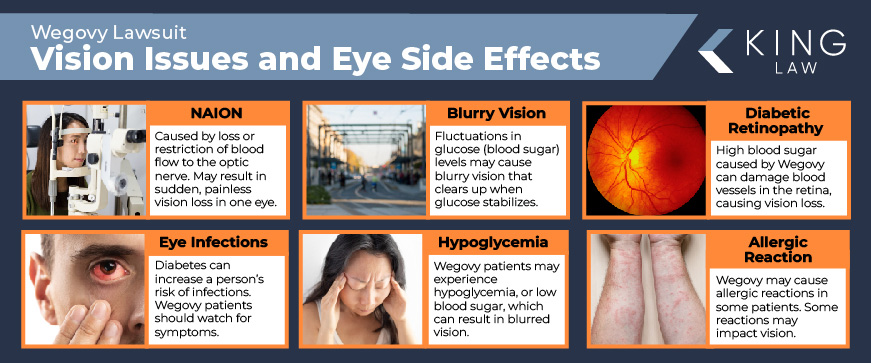
- Nonarteritic Anterior Ischemic Optic Neuropathy (NAION): A condition caused by the loss of blood flow or restricted blood flow to the optic nerve. NAION may result in sudden, painless vision loss in one eye.
- Blurry Vision: Blurry vision may be caused by fluctuations in blood sugar (glucose) levels. Symptoms may resolve as blood sugar levels normalize.
- Diabetic Retinopathy: Diabetic retinopathy is often a result of poorly managed diabetes. High blood sugar can damage the blood vessels in the retina. Left untreated, the damage may result in vision loss. While there is not a direct link between Wegovy and this condition, its role in managing diabetes is critical.
- Increased Risk of Eye Infections: Diabetes can increase a person’s risk of infections, including eye infections. Wegovy patients should pay attention to symptoms of infections, such as redness, pain, and vision changes.
- Hypoglycemia-Related Vision Issues: Patients taking Wegovy may experience hypoglycemia or low blood sugar. The condition can result in blurred vision but should resolve as blood sugar levels return to normal.
- Potential Allergic Reactions: In rare cases, Wegovy patients may experience serious allergic reactions marked by swelling or itching around the eyes.
Patients taking Wegovy, particularly those with a history of eye issues, should engage in regular eye exams to monitor and manage potential eye-related side effects. Additionally, regular blood sugar monitoring and management are critical to help minimize the risk of serious eye problems that may be associated with the drug.
Nonarteritic Anterior Ischemic Optic Neuropathy (NAION)
Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) is a serious eye condition that may cause sudden, although painless, vision loss in one eye. The condition generally occurs without warning and causes optic nerve damage due to ischemia. The optic nerve transmits visual information from the eye to the brain. NAION causes a reduction in blood flow to the optic nerve, resulting in impaired vision.
Risk factors for NAION:
- Age (usually affects people over 50)
- Diabetes
- Hypertension
- High cholesterol
- Sleep apnea
- Anatomical predispositions (such as a small cup-to-disc ratio)
Symptoms of NAION include sudden vision loss in one eye (ranging from mild to severe), missing portions of vision (visual field defects), and disc swelling. Research suggests that the fluctuations in blood pressure and blood sugar, managed by Wegocy, may reduce blood flow to organs, such as the optic nerve. Diabetic patients are also at an increased risk for vascular problems and hypoglycemia, which can contribute to conditions like NAION.
While Wegovy has not been known to cause NAION directly, its effects on blood sugar and potential impacts on vascular health in diabetic patients may increase a person’s risk of developing the condition. Individuals afflicted by the condition who are taking Wegovy should speak with an attorney immediately.
Eye Floaters (Myodesopsias)
Eye floaters (myodesopsias) are small, dark, shadowy shapes that occur in the field of vision as a result of your fluid changing in thickness. Eye floaters may appear as dots, lines, cobwebs, or other shapes and are most noticeable against bright, plain backgrounds, such as a clear sky or white wall.
Causes of Eye Floaters:
- Vitreous Shrinkage: The jelly-like substance inside the eyes can shrink and become more liquid with age. Changes can lead to clumps or strands that may cast shadows on the retina.
- Uveitis: Floaters may also be caused by inflammation of the uvea (middle layer of the eye).
- Bleeding in the eye: Bleeding in the vitreous due to diabetes, hypertension, or injury can result in floaters.
- Retinal Tears or Detachment: Retinal tears or detachment of the retina may cause floaters.
- Eye medications: Eye medications that are injected into the vitreous can lead to floaters.
A recent study also indicates that Wegovy may be linked to eye floaters. While more research needs to be done, it is suggested that factors such as diabetic retinopathy and blood sugar fluctuation may be a part of the connection between the two.
Links between Wegovy and Eye Floaters:
- Blood Vessel Damage: Poorly controlled diabetes can lead to diabetic retinopathy, damaging retinal blood vessels.
- Blood Sugar Fluctuations: Rapid or inadequate blood sugar regulation by Wegovy might stress retinal vessels, causing bleeding or changes that manifest as floaters.
- Low Blood Sugar: Episodes of low blood sugar (hypoglycemia) caused by Wegovy can lead to temporary visual disturbances, including floaters, due to changes in retinal blood flow or temporary retinal ischemia.
- Rare Inflammatory Reactions: Medications can trigger inflammation in the body, including the eyes, potentially contributing to floaters through uveitis.
- Drug Interactions: Wegovy might interact with other medications, leading to side effects that affect the eyes. Additionally, medications impacting blood clotting or vascular integrity might contribute to vitreous changes or bleeding, causing floaters.
Dry Eye Syndrome
Dry eye syndrome or dry eye disease is a common condition that occurs when the eyes fail to produce enough tears or when tears evaporate too quickly. The lack of tears can lead to inflammation and damage to the surface of the eye.
Symptoms of Dry Eye:
- Stinging, burning, or scratchy sensation in the eyes.
- Light sensitivity.
- Stringy mucus in or around the eyes.
- Redness of the eyes.
- Foreign body sensation or the feeling of having something in your eye.
- Trouble wearing contact lenses or driving at night.
- Watery eyes.
- Blurred vision or eye fatigue.
One of the most common causes of dry eye syndrome (keratoconjunctivitis sicca) is decreased tear production resulting from age, medical conditions (diabetes, rheumatoid arthritis, thyroid disorders), and certain medications. Other causes include increased tear evaporation, mainly resulting from environmental factors like wind, smoke, dry air, and an imbalance in tear composition.
Wegovy is frequently used to manage blood sugar levels in patients with Type 2 diabetes. While dry eye syndrome is not commonly listed as a side effect, it may be linked indirectly. The prescription drug may cause nausea, vomiting, and diarrhea, leading to dehydration and a reduction in tear production. In addition, diabetes is a risk factor for dry eyes, resulting from blood sugar fluctuations that can affect tear production and corneal health.
Finally, since Wegovy mimics the GLP-1 hormone, it may influence bodily fluid and potentially impact tear production. In rare cases, medications may trigger systemic inflammation, which may also affect the ocular surface and contribute to dry eye symptoms.
Eyelid Twitching
Some patients taking Wegovy may experience eye twitching, a condition known as eyelid twitching or myokymia. The condition is marked by a repetitive, uncontrollable spasm of the eyelid muscles. It most often affects the upper lid but can also occur in the lower lid. In most cases, it is harmless and temporary but can be annoying and disruptive.
Symptoms of Eye Twitching:
- Involuntary, repetitive spasms of the eyelid.
- Typically affects one eye but can occur in both.
- Lasts for a few seconds to a minute and can recur over a period of days or weeks.
- Usually, it’s not painful but can be bothersome.
Eye twitching may be caused by several factors, including stress, fatigue, caffeine, eye strain, dry eyes, nutritional imbalances, and allergies. New research suggests that Wegovy may also cause it or at least be indirectly linked to the condition. While the exact reason is unclear, it is believed that Wegovy’s tendency to cause gastrointestinal side effects such as nausea, vomiting, and diarrhea.
These gastrointestinal side effects may lead to electrolyte imbalances, particularly low magnesium levels, which can trigger muscle spasms, including eye twitching. It can also lead to dehydration and blood sugar fluctuations which may result in muscle spasms and may affect nerves throughout the body.
Other Reported Vision Issues From Wegovy
In addition to the aforementioned vision problems, Wegovy use may also lead to blurry vision, diabetic retinopathy, ocular migraines, conjunctivitis, and macular edema.
Vision issues associated with Wegovy:
- Blurry Vision: Occurs when fluctuations in blood sugar levels affect the lens shape, causing temporary vision changes. Initial changes in glucose levels from Wegovy use might cause temporary blurry vision until levels stabilize.
- Diabetic Retinopathy: Diabetic retinopathy is a complication of diabetes where high blood sugar levels damage the retina’s blood vessels. While Wegovy helps manage blood sugar levels, the risk remains if levels are not well-controlled, making regular eye exams crucial.
- Macular Edema: Marked by swelling in the macula, the central part of the retina responsible for detailed vision. Poorly controlled diabetes can cause fluid leakage into the macula. Wegovy can help manage blood sugar but cannot eliminate the risk if diabetes is inadequately controlled.
- Allergic Reactions: Although rare, an allergic reaction can cause redness, swelling, itching, and tearing in the eyes. An allergic reaction to Wegovy could manifest in the eyes, similar to other allergic reactions in the body.
- Visual Disturbances: May cause changes in color perception, seeing flashes of light, or other unusual visual phenomena. These disturbances could result from blood sugar fluctuations, blood pressure changes, or other systemic effects of Wegovy.
- Ocular Migraines: Migraines involving visual disturbances such as flashing lights, zigzag patterns, or temporary vision loss. Potentially triggered by stress, hormonal changes, or medications affecting blood vessels, though the exact cause is unclear.
- Conjunctivitis (Pink Eye): Inflammation or infection of the conjunctiva, the clear tissue covering the white part of the eye and the inside of the eyelids. While not directly linked to Wegovy, changes in immune function or systemic health while on the medication could increase susceptibility to infections or inflammation.
New JAMA Study Links Wegovy to NAION
A JAMA study published on July 3, 2024, on the “Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide” found an increased risk of the condition in patients taking semaglutide compared to those taking non-GLP-1 RA medications. The study, a retrospective matched cohort study, of 16,827 patients evaluated by neuro-opthalmologists over the course of six years (2017-2023). Patients were divided into two groups: those with type 2 diabetes and those who were overweight or obese. The groups were further subdivided based on whether they were taking semaglutide or a non-GLP-1 RA medication.
In the type 2 diabetes group, the sample size was 710 patients. 194 of those patients were taking semaglutide versus 516 who were on non-GLP-1 RA medications. The incidence of NAION was 17 events in the semaglutide group compared to 6 in the non-GLP-1 RA group. The cumulative incidence was 8.9% for semaglutide and 1.8% for the non-GLP-1 RA group over 36 months. The hazard ratio was 4.28 (95% CI, 1.62-11.29; P < .001).
In the overweight/obese group, the sample size was 979 patients. 361 of those patients were on semaglutide, and 618 were on non-GLP-1 RA medications. The NAION incidence was 20 events in the semaglutide group compared to 3 in the non-GLP-1 RA group. The cumulative incidence was 6.7% for semaglutide versus 0.8% for the non-GLP-1 RA group over 36 months. The hazard ratio was 7.64 (95% CI, 2.21-26.36; P < .001).
The study was the first to report an association between semaglutide and a higher risk of NAION, although further studies (such as a larger, multicenter study or prospective clinical trials) are required to confirm findings and determine causality. The study is bolstered by the fact that it used a large sample size and there was a manual review by experienced neuro-ophthalmologists. Furthermore, there was a propensity score matching to reduce bias.
Novo Nordisk’s Response to JAMA Study and Allegations
In an emailed statement, Wegovy manufacturer Novo Nordisk countered that there were several limitations of the JAMA study design, which was not a randomized controlled trial, as reported by Reuters. The manufacturer claimed that there was not sufficient data to establish a causal connection between the use of a GLP-1 receptor agonist (semaglutide) and NAION. The company further emphasized that the eye condition is “not an adverse drug reaction” for the marketed formulations of semaglutide.
In addition to not being a non-randomized controlled trial, criticisms of the study include that the published data was not sufficient to establish a causal association between GLP-1 receptor agonist use and NAION.
Percentage of Wegovy Users Reporting Vision Problems
The JAMA study found semaglutide users had a four times higher risk of developing NAION when being used to treat type 2 diabetes and seven times higher risk when used for treating obesity. Of the 710 patients with Type 2 diabetes studied, the NAION incident was 8.9% for those taking semaglutide compared to 1.8% for those taking another kind of medication. For the obesity treatment group, there was a 6.7% NAION incidence for patients taking semaglutide compared to a 0.8% incidence rate for patients taking other medications for weight loss.
How to File a Wegovy Vision Loss Lawsuit
In order to file a Wegovy vision loss lawsuit, you must meet certain eligibility requirements. The best way to determine whether you meet these qualifications is to consult with an attorney. An attorney can provide a free initial consultation to determine the validity of your case.
Steps to file a Wegovy vision loss lawsuit:
- Determine eligibility: The first step in a Wegovy vision loss lawsuit is to determine whether you meet the eligibility requirements. Attorneys generally offer a free case review to verify lawsuit eligibility.
- Collect evidence: If eligibility requirements are met, you will need to collect the evidence necessary to substantiate your claim. Evidence may include medical records, proof of prescription, and witness testimonies. A Wegovy lawsuit attorney can help review medical records and usage history as well as provide guidance on the legal process.
- File the lawsuit: With the appropriate evidence, the case can be filed. It is important to adhere to statutes of limitations or state-specific deadlines when filing. An attorney can ensure that all legal requirements are met and that the case is filed in the appropriate courthouse.
- Negotiate a settlement: Throughout the case, an attorney will negotiate with the other party to attempt to reach a fair and full settlement.
- Prepare for trial: If a settlement cannot be reached, the matter will be set for trial. Both sides will present their case. If the outcome is not favorable, the decision may be able to be appealed.
If you have been diagnosed with NAION or another eye problem after taking Wegovy, you may be eligible to take legal action. It is imperative to supply your attorney with all of your health information and supporting documentation to ensure they can make an accurate assessment of the case. Once an attorney is retained, they can represent you throughout the process and file the claim on your behalf.

Contact a Wegovy Vision Loss Lawyer
Were you diagnosed with NAION after taking Wegovy? A new study has shown an increased risk of the eye condition for semaglutide users. Affected individuals may be entitled to take legal action and are strongly encouraged to contact the Wegovy vision loss lawyers at King Law. Our legal team has extensive experience handling these cases. Contact our office today to schedule a free consultation.
Frequently Asked Questions (FAQs)
Find answers to any major questions you have regarding the Wegovy vision loss lawsuits below.

