
Trulicity Lawsuit Overview
Trulicity, a once-weekly injectable used to treat type 2 diabetes in adults and children 10 years of age and older, has been the subject of a number of lawsuits. The drug is also used off-label for weight loss, although it has not received FDA approval for this use. The lawsuits allege that the drug, which mimics the GLP-1 hormone in the body that aids in insulin secretion, causes severe side effects, including gastroparesis and pancreatic cancer.. Legal documents also suggest that the manufacturer of Trulicity, Eli Lilly, knew of these risks and failed to adequately warn healthcare providers or patients of the dangers.
Eli Lilly’s lack of transparency may have violated legal and ethical obligations, influencing patient treatment decisions. Currently, there are over hundreds of lawsuits that have been centralized in multidistrict litigation against the manufacturers of GLP-1 receptor agonists. Patients who suffered adverse gastrointestinal issues after taking Trulicity may qualify for compensation by taking legal action. It is strongly recommended that affected individuals contact King Law to schedule a free, no-obligation consultation.
King Law is currently investigating Trulicity lawsuits involving a diagnosis of:
- Gastroparesis/stomach paralysis
- Gastrointestinal Obstruction/ileus
- Blindness(NAION) and/or vision problems
- Daily vomiting lasting at least 3 weeks
- Blood clots/deep vein thrombosis (DVT) or pulmonary embolism (PE)
Individuals are encouraged to contact our office to discuss legal options in the Trulicity lawsuit. Consultations are provided free of charge and without obligation to retain our services.
Trulicity Lawsuit Update – 2025 Update
April 16, 2025: Parties in Trulicity Lawsuit to Hold Hearing on Gastroparesis
On May 14, 2025, the parties involved in the Trulicity multidistrict litigation will hold a hearing about the reliable diagnostic process for gastroparesis. Gastroparesis is a central issue in the Trulicity and GLP-1 case, because it is one of the dangerous complications people are experiencing after taking those products. The plaintiffs and the defendant corporations will interview experts, like doctors, who can talk about Trulicity, GLP-1s, and gastroparesis. The plaintiffs hope to uncover information to support their legal claims against Eli Lilly and other pharmaceutical corporations.
April 2, 2025: Lawsuits Against Eli Lilly’s Trulicity Increase in March
There are now 1,685 active lawsuits pending against Trulicity’s maker Novo Nordisk and other GLP-1 manufacturers. These lawsuits are part of the group, federal litigation for people harmed by GLP-1 drugs. Many people have taken Trulicity to treat their type 2 diabetes and then experienced serious side effects from the drug. Lawsuits against Trulicity and other GLP-1 drugs are expected to increase over the coming months.
March 25, 2025: Food & Drug Administration Says Trulicity Shortage Still Active as Lawsuits Increase
On February 21, 2025, the Food & Drug Administration lifted many of the semaglutide injection shortages; however, it clarified that the dulaglutide shortage is still in effect. Trulicity is a dulaglutide injection drug used to treat diabetes and cardiovascular disease and help people lose weight. Many people taking Trulicity and other GLP-1 drugs—including Ozempic—have developed severe gastrointestinal symptoms and other serious conditions after taking it as prescribed. This ruling from the FDA means people can still get compounded forms of Trulicity, which pose similar health risks to the brand-name drug.
March 4, 2025: Hearing on Expert Witnesses Approaches as New Trulicity Cases Are Filed
On May 14, 2025, the plaintiffs and defendants will present arguments for a hearing on what expert testimony they can use during the first round of test trials in the GLP-1 federal litigation, which includes the makers of Trulicity. Expert testimony can be compelling in these types of cases, helping to shape the plaintiffs’ arguments or refute the drug company’s claims. The multidistrict litigation against the pharmaceutical giants that made Trulicity and other GLP-1 drugs has grown to 1,521 pending lawsuits on the federal level, with more cases being filed in state courts across the country.
February 6, 2025: More Lawsuits Filed Due to Injuries from Trulicity and Other GLP-1 Drugs
There are now 1,443 lawsuits pending against Trulicity manufacturer Eli Lilly and other GLP-1 manufacturers. These lawsuits are part of a group litigation called MDL 3094 In Re: Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAS) Products Liability Litigation, which represents people harmed by GLP-1 drugs. As more people suffer stomach paralysis, bowel blockages, and other serious side effects associated with Trulicity, more people will join this group litigation as a way to be compensated.
December 2, 2024: More People Alleging Injuries From Trulicity Join Federal Lawsuit
People who experienced injuries after taking Trulicity and other GLP-1 drugs are continuing to file lawsuits against GLP-1 manufacturers. As of December 2, 2024, there are 1,300 pending lawsuits in MDL 3094, Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation. This multidistrict lawsuit has many plaintiffs who allege their gastrointestinal disorders, blood clots, and vision loss was caused by using Trulicity and other GLP-1 drugs. It is likely more people will join the MDL throughout 2025.
November 22, 2024: Researches Push For More Studies on Trulicity and Other GLP-1 Drugs and Cancer Risks
A study published in the journal Endocrine Connections urged the scientific community to examine whether or not GLP-1 drugs, like Trulicity, could increase the risk of someone developing thyroid cancer. The study, titled “Glucagon-like peptide 1 receptor agonists and thyroid cancer: is it the time to be concerned?” examined data from people who were on GLP-1 drugs for 1 to 3 years. They found these people had a 58% increased risk of developing thyroid carcinomas. They called for more research into the possible carcinogenic qualities of GLP-1 drugs.
November 7, 2024: Deadline Set To File Expert Reports in Trulicity Lawsuit
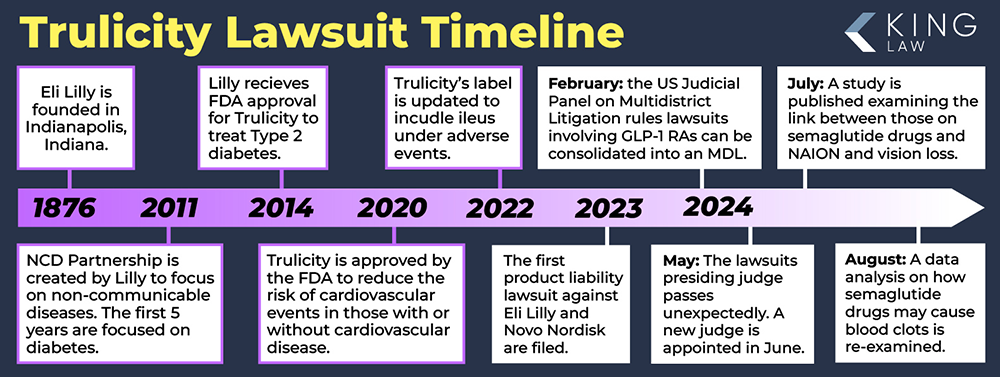
The judge in the Trulicity multidistrict litigation (MDL 3094) issued an order that set a deadline for submitting expert reports. Judge Karen Spencer Marston issued an order that set the deadline for Plaintiff expert reports to be submitted by November 18, 2024. The deadline for defendants to submit their expert reports is December 23, 2024. Both sides could file an extension. Expert reports are written statements from expert witnesses. This information is used throughout the trial.
October 4, 2024: Lawsuits Against Eli Lilly Escalate as Trulicity Side Effect Concerns Grow
The number of lawsuits targeting GLP-1 drug manufacturers, including Eli Lilly, the maker of Trulicity, saw a 20% increase between August and September. As of October 1, 2024, there were 1,090 cases pending in MDL 3092. Increasing reports of severe side effects from users of GLP-1 agonist drugs, like Trulicity, have likely driven this rise in cases. With more Americans taking these medications, legal experts predict tens of thousands of additional lawsuits to be filed in the coming months.
August 6, 2024: Discovery Process Defined in Trulicity Lawsuit by Judge Marston
A 19-page order was recently issued by Judge Marston covering how discovery will proceed in the Trulicity Lawsuit. The first major step in the GLP-1 litigation is the request for millions of documents to be turned over by Eli Lilly and Company. The judge requested that electronic data be maintained and all versions of emails should be produced to the court. Additionally, the order notes that both parties should discuss how text messages, internal communications, or social platforms might have been used to maintain communications or discoverable material. The parties often have many disagreements about what should be produced. Plaintiffs will want more information while defendants will want to produce less. This is a critical development in the Trulicity lawsuit and will likely take several months to complete.
August 2024: A data analysis examining how semaglutide drugs in the GLP-1 agonist family might cause blood clots is being re-examined. The study, published in the Endocrine Journal, saw a 266% increase in deep vein thrombosis (DVT) in people taking semaglutide drugs. The medical community will be tracking whether or not these same dangerous side effects apply to other GLP-1 drugs. If they do, that means dulaglutide, the active ingredient in Trulicity, could cause blood clots and DVT.
July 2024: A study that examined the link between taking semaglutide drugs and suffering eye strokes is in the news. JAMA’s Ophthalmology published findings that people taking semaglutide drugs, like Ozempic and Wegovy, may suffer vision loss. The drugs may cause a type of eye stroke called nonarteritic anterior ischemic optic neuropathy (NAION). Medical professionals will be paying close attention to see if this same dangerous side effect is present in other GLP-1 agonist drugs, like Trulicity.
June 2024: The honorable Karen Spencer Marston is appointed to preside over the GLP-1 receptor agonist cases consolidated into an MDL out of the Eastern District of Pennsylvania.
May 2024: The judge presiding over MDL 3094, the honorable Gene E.K. Pratter died unexpectedly, potentially delaying further action with the process until a new judge can be assigned.
February 2024: In February 2024, the United States Judicial Panel on Multidistrict Litigation (MDL Panel), agreed to consolidate lawsuits involving glucagon-like peptide-1 receptor agonists (GLP-1 RAs), including Trulicity. MDL 3094 is being heard in the Eastern District of Pennsylvania and involves lawsuits with common questions of fact including whether the manufacturers (Eli Lilly and Novo Nordisk) knew or should have known that their products cause severe gastrointestinal injuries such as gastroparesis.
About the Trulicity Lawsuit:
What Is Trulicity and How Does It Work?
Trulicity vs Ozempic: Comparing Their Similarities and Differences
Trulicity Side Effects and Symptoms
Trulicity Long-Term Side Effects
Studies Link Trulicity to Gastroparesis and Bowel Obstruction
Trulicity Manufacturer: Eli Lilly and Company
Eli Lilly’s Misleading Marketing Strategy Promoting Trulicity
Trulicity FDA Approval and Its Black Box Warning
Trulicity Active and Inactive Ingredients
Eligibility Criteria for Filing a Trulicity Lawsuit
Recoverable Damages in a Lawsuit Against Trulicity
What Is Trulicity and How Does It Work?
Trulicity is a GLP-1 receptor agonist manufactured by Eli Lilly & Company. The active ingredient in the incretin mimetic is dulaglutide, which shares 90% of the amino acid sequence with naturally occurring GLP-1. It received approval from the U.S. Food and Drug Administration (FDA) for the treatment of type 2 diabetes in adults and children 10 years of age and older in conjunction with a healthy diet and exercise. It is frequently used to lower the risk of major cardiovascular events in adults with type 2 diabetes who have existing heart disease or risk factors.
Trulicity mimics GLP-1, stimulating insulin secretion when blood glucose levels are high, reducing glucagon secretion from the liver, and slowing emptying. Because the drug binds to GLP-1 receptors in the brain, regulating appetite and reducing food intake, the once-weekly subcutaneous injectable is sometimes prescribed off-label for weight loss. It can also help lower and control blood sugar levels by enhancing insulin release and reducing the speed at which sugar is absorbed into the bloodstream.

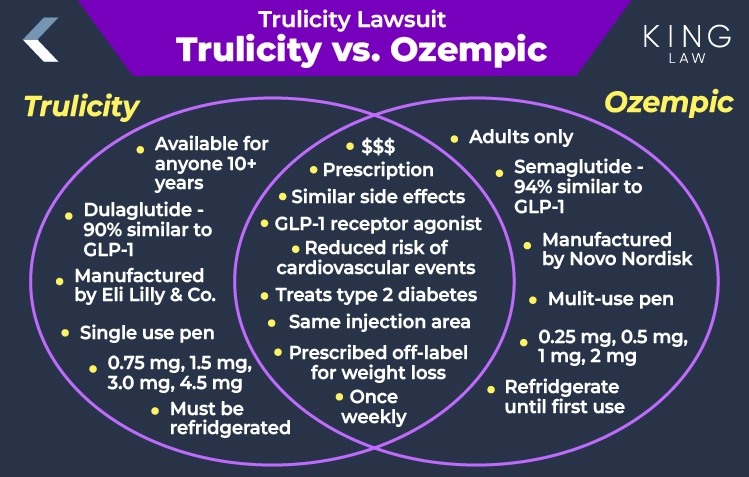
Trulicity vs Ozempic: Comparing Their Similarities and Differences
While Trulicity and Ozempic are prescription drugs belonging to a group of medications known as GLP-1 receptor agonists used to treat type 2 diabetes that can reduce the risk of major cardiovascular events in adults with this conduction who have existing heart disease or risk factors, they have a number of differences.
Differences between Trulicity and Ozempic:
- FDA approval and age suitability: Both medications are available by prescription only and used to manage blood sugar levels in conjunction with diet and exercise. Trulicity, however, is approved for treatment of type 2 diabetes in adults and children ages 10 and older, while Ozempic is approved for treatment of type 2 diabetes in adults only.
- Administration and packaging: While both drugs require once weekly injections, Trulicity is only available in single-use pens (each pen is used one and then discarded) that should be stored in the refrigerator. Ozempic, on the other hand, is distributed in multi-use pens which can be used multiple times with disposable needles and should be stored in the refrigerator but can be kept at room temperature after first use.
- Dosages and injection sites: Trulicity is available in dosages of 0.75 mg, 1.5 mg, 3 mg, and 4.5 mg. Ozempic is available in dosages of 0.5 mg, 1 mg, and 2 mg. The drugs may be administered subcutaneously in the stomach, thigh, or upper arm.
- Mechanism of action: Both drugs fall into the class of medications known as GLP-1 receptor agonists that enhance insulin secretion, suppress glucagon release, and slow gastric emptying to help control blood sugar levels.
- Active ingredient: The active ingredient in Trulicity is dulaglutide which is 90% similar to human GLP-1. The active ingredient in Ozempic is semaglutide, which is 94% similar to human GLP-1. Both drugs can influence appetite by acting on GLP-1 receptors in the brain.
While neither drug is officially approved by the FDA for weight loss, they are frequently used off-label for this purpose. Additionally, both medications cause a delay in gastric emptying, which can result in stomach paralysis (gastroparesis), intestinal blockage (ileus), and other severe gastrointestinal side effects.
Trulicity Side Effects and Symptoms
There are a number of known side effects of Trulicity. While many of the side effects are common and mild, such as nausea, vomiting, and diarrhea, some are more severe resulting in long-term health problems.
Common side effects of Trulicity:
- Nausea
- Vomiting
- Diarrhea
- Stomach pain
- Decreased appetite
- Indigestion
- Fatigue
- Weight loss
- Injection site reactions, such as redness and irritation
In most cases, mild side effects improve as the body adjusts to the medication, however, more serious conditions may require medical intervention to resolve. These conditions may also be managed with lifestyle adjustments like diet changes in many instances.
Potentially severe side effects of Trulicity:
- Hypoglycemia
- Gastroparesis (stomach paralysis)
- Ileus (intestinal blockage)
- Gallbladder disease
- Kidney damage
- Pancreatitis
- Thyroid tumors (Trulicity carries a boxed warning of thyroid tumors after studies showed an increased risk in animals, but had unclear implications for humans)
- Allergic reactions
Signs and symptoms of serious side effects may include severe abdominal pain, persistent nausea, vomiting, fever, dark urine, or signs of an allergic reaction. Long-term risks of Trulicity include the potential worsening of or new onset kidney damage and severe digestive problems such as gastroparesis or ileus. Some conditions may require dose adjustments, stopping Trulicity, or emergency medical care.
Trulicity Long-Term Side Effects
Litigation indicates that Trulicity may have a number of long-term side effects with prolonged use. Any patient taking Trulicity should engage in regular monitoring for symptoms.
Long-term side effects associated with Trulicity:
- Thyroid Cancer: Trulicity has a boxed warning (the most serious warning issued by the FDA) due to the drug’s potential for developing thyroid tumors, including cancer. Symptoms to monitor include neck lumps, swallowing difficulties, hoarseness, and shortness of breath.
- Hypoglycemia: When taking Trulicity low blood sugar levels can occur, particularly when paired with other diabetes medications.
- Kidney Problems: Patients may be at risk of acute kidney issues, which could require medical intervention.
- Gallbladder Disease: Trulicity may increase a person’s risk of developing conditions like cholecystitis (gallbladder inflammation) and gallstones, which may require medical attention.
- Pancreatitis: Taking Trulicity may cause an inflammation of the pancreas, a serious condition that demands immediate medical care.
- Gastroparesis: Lawsuits allege that the slow stomach emptying caused by Trulicity may lead to stomach paralysis which can cause severe discomfort and nutritional issues.
- Ileus and intestinal blockage: Obstruction in the intestines that can cause severe pain and require surgical intervention.
It is strongly recommended that individuals experiencing adverse health conditions after taking the medication contact their healthcare providers and consider consulting legal counsel
Studies Link Trulicity to Gastroparesis and Bowel Obstruction
There have been several studies that point to a link between the use of Trulicity and severe gastrointestinal issues such as gastroparesis (stomach paralysis) and bowel obstruction (ileus). Individuals who experience these serious side effects should consult with their healthcare professional and consider consulting an experienced attorney.
Research indicating a link between Trulicity and severe side effects:
- Gastroparesis: Studies show a connection between the use of Trulicity and an increased risk of developing gastroparesis or delayed stomach emptying.
- A case report of a 57-year-old woman who was taking dulaglutide (the active ingredient in Trulicity) to treat type 2 diabetes presented with abdominal bloating, nausea, and vomiting. A gastric emptying study (GES) showed delayed gastric emptying. The condition was gradually resolved once the patient stopped taking the drug.
- A review of Diabetic Gastroparesis found that the class of drugs known as glucagon-like peptide 1 (GLP-1) receptor agonists such as dulaglutide (Trulicity) may “exacerbate the symptoms of diabetic gastroparesis” and therefore are not recommended for individuals experiencing symptoms of gastroparesis.
- A 2023 study out of the University of British Columbia linked GLP-1 agonists to a “heightened risk of severe gastrointestinal problems.” These problems include gastroparesis, pancreatitis, and bowel obstruction. The findings, which showed that these events were rare but significant, particularly in patients using the drugs for weight loss, were published in the Journal of the American Medical Association (JAMA).
- Bowel Obstruction: Studies show a link between Trulicity and an increased risk of bowel obstruction, particularly for those new to the medication (had been taking the drug for less than one month).
- A 2020 report in the Journal of the Endocrine Society indicated that dulaglutide (Trulicity) may cause small bowel obstruction. In 2017, eight cases were reported with most of them requiring surgical intervention.
It is recommended that patients taking Trulicity or another GLP-1 agonist, weigh the benefits of using the medication against the potential risks, particularly when using the drug for a non-diabetic condition such as weight loss.
Trulicity and Blood Clots
Some GLP-1 agonist drugs have been tied to an increased risk of a type of blood clot called a deep vein thrombosis. A study published in the Endocrine Journal found people who took semaglutide drugs to treat their type 2 diabetes were about 3.5 times more likely to develop DVTs. DVT is the leading cause of pulmonary embolisms, which can cause organ damage or death.
The active ingredient in Trulicity is dulaglutide, which is different than semaglutide. However, both drugs belong to the same class of drugs, called GLP-1 agonists. More patient studies will be needed to determine if dulaglutide drugs, like Trulicity, increase the risk of blood clots in their users.
Trulicity and Vision Loss
Trulicity is part of a class drugs called GLP-1 agonists. This class of drugs includes semaglutides like Ozempic and Wegovy. Semaglutide drugs have been shown to increase the risk of eye strokes that cause vision loss.
A study that was published in JAMA’s Ophthalmology noted a significant increase NAION in people taking semaglutide drugs compared to people receiving other treatments for their type 2 diabetes. NAION stands for nonarteritic anterior ischemic optic neuropathy. NAION is the second most common form of optic neuropathy and a common cause of adult blindness. More studies will be needed to see if other GLP-1 drugs, such as Trulicity, carry an increased risk of vision loss.
Trulicity Manufacturer: Eli Lilly and Company
Trulicity is manufactured by pharmaceutical giant Eli Lilly & Company. The medicine company is headquartered in Indianapolis, Indiana. Founded on May 10, 1876, Eli Lilly now has over 44,000 employees and markets its products in over 100 countries around the world.
On September 18, 2014, Eli Lilly received FDA approval for TRULICITY®. It was noted as being the first FDA-approved type 2 diabetes medication to reduce major adverse cardiovascular events (MACE) in adults. It is approved for both primary and secondary prevention of major cardiovascular events for people with type 2 diabetes. At one point, Trulicity was the most prescribed GLP-1 receptor agonist (RA) in the United States.
Eli Lilly’s Misleading Marketing Strategy Promoting Trulicity
Since Trulicity’s launch in 2014, there have been a number of concerns about Eli Lilly’s marketing strategy. The FDA reportedly contacted the manufacturer suggesting that the drug had not been marketed in a truthful and non-misleading way.
A 2022 warning letter from the Office of Prescription Drug Promotion (OPDP) highlighted an Instagram ad posted by the company that reportedly made, “false or misleading claims and representations about the benefits and risks of Trulicity.” It found that the company minimized the risks and overemphasized the benefits of the drug. The letter also detailed prior communications sent by the FDA to the manufacturer about their marketing efforts.
Current litigation alleges that Eli Lilly used an unconventional launch strategy by focusing on payers rather than targeting physicians directly and that they used sales representatives to emphasize Trulicity’s benefits to insurance providers and payers. The company also implemented a direct-to-consumer campaign, utilizing print, digital, and television ads to inform patients about the drug’s benefits.
These advertisements highlighted Trulicity’s ease of use, long-lasting effects, and A1C impact as well as promoting the non-insulin nature of the drug and the convenience of the pen’s hidden needle with the “Click to activate your within,” tagline.
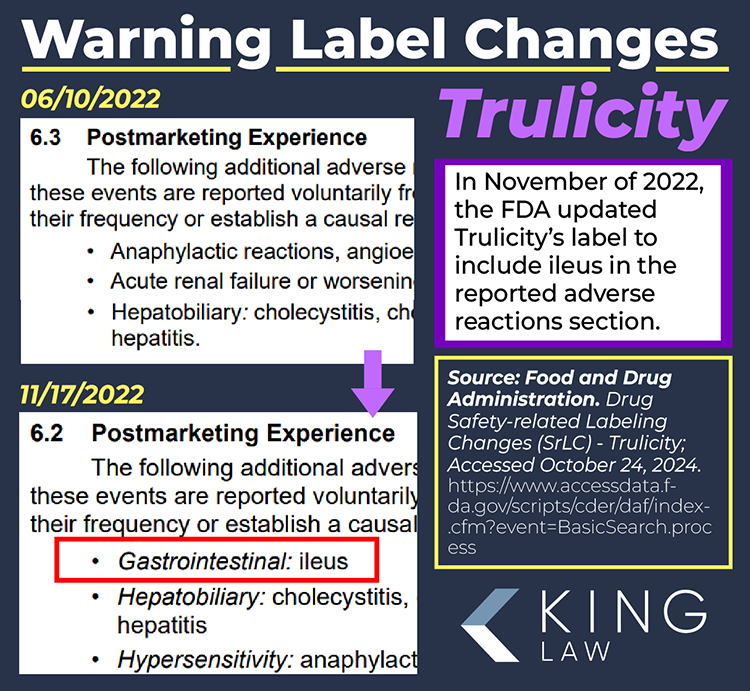
Trulicity FDA Approval and Its Black Box Warning
Trulicity carries a “boxed warning” (formerly black box warning) regarding the risk of thyroid C-cell tumors. A boxed warning is the highest level of alert issued by the FDA. According to the warning, dulaglutide (the active ingredient in Trulicity) was found to cause an increased risk of thyroid C-cell tumors in male and female rates. The direct implication for similar effects in humans; however, is noted but remains uncertain.
The boxed warning also mentions the risk of pancreatitis and that the medication is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) and patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Patients are advised to monitor for symptoms of thyroid tumors, including swelling of the neck, difficulty swallowing, or persistent hoarseness.
Trulicity Active and Inactive Ingredients
The active ingredient in Trulicity is dulaglutide with strengths of 0.75 mg and 1.5 mg per 0.5 mL. The medication comes in multiple packages include: four syringes per carton (NDC:0002-1433-80), one syringe per package, prefilled biologic delivery system (NDC:0002-1433-01), and two syringes per carton (NDC:0002-1433-61).
Inactive ingredients in Trulicity:
- Trisodium Citrate Dihydrate (1.37 mg in 0.5 mL): Often used as a buffering agent or pH adjuster. It helps stabilize the pH of the solution to make it more suitable for injection.
- Citric Acid Anhydrous (0.07 mg in 0.5 mL): This ingredient also serves as a pH adjuster, helping to maintain the stability and effectiveness of the active ingredient by keeping the solution acidic.
- Mannitol (23.2 mg in 0.5 mL): Used primarily as a tonicity agent, mannitol helps adjust the osmolarity of the injection solution to be compatible with body fluids. It can also serve as a stabilizer or bulking agent.
- Polysorbate 80 (0.10 mg in 0.5 mL): This is a surfactant and emulsifier, which helps to stabilize and dissolve the active ingredient in the solution. It can also be used to prevent surface adsorption of the drug to the container.
- Water for Injection: Used in a quantity sufficient to make up the volume. This is the solvent in which all the ingredients are dissolved. It is specially purified and sterile water used for making injectable solutions.
Each syringe of Trulicity is a Type 3, prefilled Biologic Delivery Device/System, intended for single use.
Eligibility Criteria for Filing a Trulicity Lawsuit
To file a Trulicity lawsuit, individuals must meet certain eligibility requirements. These requirements include the ability to show proof of use of the drug and diagnosis of a severe side effect related to its use. To determine if you qualify to take legal action, it is strongly recommended that you seek consultation from an attorney.
Eligibility criteria for a Trulicity lawsuit:
- Proof of use of Trulicity
- Proof of diagnosis of a severe side effect including prolonged vomiting, gastroparesis (stomach paralysis), ileus (intestinal obstruction or blockage), blindness and/or vision problems, bile duct cancer, Lou Gehrig’s Disease (ALS), gallbladder cancer, or sinus cancer.
It is important to note that not all individuals who experienced side effects after taking Trulicity will qualify for a lawsuit. An attorney can help review your case to determine its validity during an initial consultation. If successful, a legal claim may result in compensation for medical expenses, lost wages, and more.

Evidence to Gather to File a Lawsuit
- Medical records
- Prescriptions
- Emergency room visits
- Consultations with specialists
- Doctor’s notes
- Expert testimony
- Proof of diagnosis
- Evidence of lost wages
Recoverable Damages in a Lawsuit Against Trulicity
Individuals with viable claims against the manufacturer of Trulicity, Eli Lilly, may be entitled to compensation for economic and non-economic losses.
Recoverable damages in a Trulicity lawsuit may include compensation for:
- Medical Expenses: Coverage for both past and future medical bills incurred due to Trulicity-related conditions.
- Pain and Suffering: This may include compensation for physical pain, emotional distress, and reduced quality of life both before and after treatment.
- Lost Wages: Individuals may be entitled to lost earnings and diminished earning capacity due to the adverse effects of Trulicity.
- Non-Economic Damages: This includes compensation for emotional distress and loss of enjoyment of life due to the impact of the condition.
- Punitive Damages: These may be awarded in cases where Eli Lilly’s behavior is deemed egregiously harmful, aiming to punish the company and prevent future misconduct.
How to File a Trulicity Lawsuit
Individuals who have suffered adverse health conditions after taking Trulicity are encouraged to seek legal advice to determine if they may be eligible to take legal action.
Steps to filing a Trulicity lawsuit:
- Determine Eligibility: In order to determine eligibility it is advised that you seek an initial consultation with a lawyer.
- Collect Evidence: Once eligibility is determined, it is important to gather the evidence necessary to substantiate your claim. Evidence may include medical records, testimonies, and documentation.
- File the lawsuit: After evidence is collected, your attorney will file your claim in the appropriate courthouse and ensure all state deadlines are met.
- Negotiations and court proceedings: Once the case is filed, your attorney may enter into negotiations with the defense. If a fair and full settlement cannot be reached, the matter may be set for trial.
- Trial preparation: Your attorney will prepare the matter for trial if a fair settlement cannot be reached. Your case will be presented before a judge and jury.
Trulicity Lawsuit Statute of Limitations and Deadlines
The statute of limitations for a Trulicity lawsuit is state specific and can vary significantly by case. In addition to state law, factors that may influence the length of time a person has to file a claim include the date that they discovered their Trulicity-related injuries and their age.
Trulicity Settlement and Payout Amounts
Trulicity settlement and payout amounts are expected to vary substantially depending on the individual circumstances of the case. While there is no standardized settlement amount, the anticipated range for serious injuries is expected to be between $400,000 and $700,000. The extent of an individual’s losses and their injuries may significant;y influence the final payout.
An experienced attorney can help in maximizing compensation. It often takes an awareness of the full range of damages and documented evidence to ensure aggrieved parties receive the compensation they deserve. Personal injury lawyers generally possess the negotiating and legal skills necessary for securing fair settlement offers.
Contact a Trulicity Lawyer
Individuals who suffered severe gastrointestinal issues, such as gastroparesis or intestinal blockage after taking Trulicity are encouraged to contact King Law. The attorneys at King Law have extensive experience handling prescription drug-related claims and can help you navigate these complex lawsuits. Contact our office today to schedule a free, no-obligation consultation.

