
Suboxone, a two-drug medication commonly used to treat opioid addiction, has come under scrutiny as lawsuits against the manufacturer of the drug mount. Court documents indicate that the sublingual strips may put users at an increased risk of developing severe dental problems, including tooth decay, gum disease, and tooth loss. Thousands of lawsuits were recently consolidated into multidistrict litigation (MDL) in the Northern District of Ohio, with thousands of additional plaintiffs expected to come forward as litigation continues.
Suboxone Lawsuit Overview
Suboxone is a two-drug medication that has become increasingly popular since its debut in 2002. Previously available only as a dissolvable tablet, the prescription now comes in sublingual strips containing a 4:1 ratio of buprenorphine and naloxone. The medication is used to treat opioid addiction and has been touted for its quick efficacy and lower risk of dependency compared to other opioid addiction treatments such as methadone.
The introduction of the fast-acting under-the-tongue strip in 2012 propelled the drug’s use. Unfortunately, the sublingual strip can cause severe dental issues, according to an increasing number of lawsuits against the manufacturer. Despite concerns, there were no warnings about the risk of these problems until 2022, nearly a decade after the initial reports were filed.
Dental problems reportedly linked to Suboxone use:
- Severe tooth decay
- Tooth loss and extraction
- Cracked teeth and increase in cavities
- Injuries to the gum and teeth
- Oral infections
- Dental caries (such as loss of enamel, dentine, etc.)
These problems have led to the need for Suboxone users to get root canals, crowns, crown replacements, and other expensive dental procedures. Allegations against the manufacturer of Suboxone, Indivior, Inc., include that the company knew about the risk of severe tooth decay associated with the drug and failed to warn prescribing doctors and patients, prioritizing profits over consumer safety. Individuals who have used Suboxone and suffered adverse reactions may be eligible to file a lawsuit or join the multidistrict litigation pending in federal court.
It is believed that Invidivor, a specialty pharmaceutical company focused on opioid dependency drugs, may have rushed the sublingual film to market, ignoring adverse event reports to avoid potential competition. Further allegations include that the manufacturer may have failed to complete their due diligence when it comes to warning consumers about the potential for severe dental issues or even intentionally hid the risks to prevent a slowdown from the U.S. Food and Drug Administration. Individuals adversely affected by the drug are encouraged to seek legal counsel. Depending on the circumstances, they may be eligible for compensation related to the psychological and emotional distress caused by the drug’s side effects and the financial burden related to costly dental treatments.
Suboxone Lawsuit for Tooth Decay – 2025 Updates
March 20, 2025: Order From Judge May Decrease Filing Fees For People Filing Suboxone Lawsuits
In the Suboxone group lawsuit, Judge Calabrese has issued an order in that will benefit many plaintiffs in the lawsuit. The March 14, 2025, order stated that up to 100 plaintiffs can be included on one complaint and that there would be only one filing fee associated with that single complaint filing. Usually, a federal court filing fee is $405, so this will save plaintiffs money at the time they file their lawsuit. Also, the order stated that electronic, bulk-uploading will be allowed for cases. This action will help the lawyers understand how many cases there are and what injuries are being cited in those cases. This will allow for settlement negotiations at the right time. It is believed that there are tens of thousands of unfiled cases, and these advances may motivate Suboxone lawsuit filings.
March 14, 2025: Case-Selection Process Underway in the Suboxone Lawsuit
Judge Calabrese issued an important order in the Suboxone group lawsuit. On March 14, 2025, he issued Case Management Order 15, which identified the selection protocol for the bellwether trials (test trials). To start narrowing down what cases will be part of the bellwether process, 500 cases will be randomly selected and their records reviewed. Then, several rounds of filtering will occur, until the first plaintiff for the test trial is submitted. This development is good news for people who were harmed by Suboxone, as it demonstrates positive progress toward test trials and settlements.
March 3, 2025: Judge in Suboxone Lawsuits Issues Order to Help Plaintiffs Get Their Dental Records
The judge in Suboxone group lawsuit, J. Philip Calabrese, issued an order to help people harmed by Suboxone films obtain their dental records. Some dentists have been slow to respond to patients’ and their lawyers’ requests for dental records, which are required to file a successful lawsuit for tooth injuries. Judge Calabrese clarified acceptable authorization for releasing dental records and stated that practices that do not reply could be held in contempt of court. The goal of this order is to ensure the timely submission of documentation as plaintiffs work toward settlements for Suboxone-related injuries.
February 25, 2025: Tolling Agreement Could Extend Time Allowed to File a Suboxone Lawsuit
The judge overseeing the group Suboxone lawsuit, Judge J. Philip Calabrese, has told lawyers representing Indivior they should consider a tolling agreement. A tolling agreement would change the legal deadline to file a Suboxone lawsuit. The judge has told the Suboxone manufacturer that if they do not extend the time deadline, plaintiffs will be forced to file all cases, regardless of the quality of those cases. This would be necessary to meet the filing deadline. It would likely be better for all parties in this lawsuit if a tolling agreement was reached and people harmed by Suboxone films had more time to file their cases.
February 11, 2025: If you haven’t found a suboxone attorney – We urge you to do so now
Many law firms, including ours, we stop taking new suboxone cases for most states this week. This is due to statute of limitations issues. Whether it is us or someone else, if you want to be part of the Suboxone lawsuit we encourage you to get signed up now.
February 3, 2025: More Than 140 New Suboxone Lawsuits Filed, Including a Lawsuit from California Woman Who Lost All of Her Teeth
The multidistrict litigation (MDL) against Suboxone’s manufacturers is growing. As of February 3, 2025, there are 896 pending lawsuits in the consolidated litigation against Suboxone, up 142 cases from January. These lawsuits have been filed by people whose teeth and lives have been damaged by Suboxone sublingual films. One of the people to join the MDL was a California woman who says she lost all of her teeth and had to endure a 15-hour dental-implant surgery. In her lawsuit against Indivior and other defendants, she is seeking multiple damages as she says she was not properly warned about the dangers of Suboxone films. Lawsuits against Suboxone manufacturers are expected to increase throughout 2025.
January 17, 2025: Dental Damages Lead to Woman Filing New Suboxone Lawsuit
A woman in New Mexico has filed a Suboxone lawsuit after its sublingual films caused permanent damage to her teeth, requiring substantial dental work. Gayle Ortega filed her lawsuit against Indivior, Aquestive Therapeutics, and Monosol Rx, who all made or sold versions of buprenorphine-naloxone strips. Ortega was prescribed Suboxone films after she became addicted to painkillers prescribed by her doctor. Her complaint said she was never warned of the dangerous dental risks from Suboxone films. Her lawsuit was filed as part of the multidistrict litigation against Suboxone film manufacturers (Suboxone (Buprenorphine/Naloxone) Film Product Liability Litigation). She is seeking multiple damages for her dental injuries, pain and suffering, loss of income, and other injuries.
January 8, 2025: Suboxone Multidistrict Lawsuit Will Proceed; Cases Are Not Dismissed
The consolidated litigation against Suboxone film manufacturer Indivior will move forward. Indivior had asked for all of the dental injury lawsuits in the multidistrict litigation (MDL) to be dismissed. This would have been bad news for many people harmed by Suboxone films. However, in an order and opinion issued by Judge J. Philip Calabrese and Magistrate Judge Jennifer Dowdell Armstrong, the Suboxone litigation will move forward throughout 2025. Although some specific allegations against Indivior have been dismissed, the bulk of the legal complaint against them was allowed by the courts. This is great news for people who suffered severe dental issues due to Suboxone films. The next case conference will be held January 14, 2025, where plaintiffs will learn more about the path forward for their cases and potential future settlements.
January 2, 2025: 38 More People With Dental Injuries Join the Consolidated Litigation Against Suboxone
From December to January 2, 2025, 38 more people who were injured by Suboxone sublingual films joined the multidistrict litigation (MDL) against the drug’s manufacturer, Indivior. As of January 2, there are 754 pending lawsuits in the Suboxone MDL, which is similar to a class action lawsuit against the makers of Suboxone films. These films have caused severe tooth decay and dental injuries in many patients. As the MDL progresses throughout 2025, people harmed by suboxone could receive payments through settlements. This assumes Indivior’s motion to dismiss the lawsuits is unsuccessful.
December 26, 2024: Motion to Dismiss Suboxone Sublingual Film Lawsuits Still Undecided
As 2024 comes to a close, the judge has not ruled on the defendants’ request to dismiss Suboxone lawsuits. Plaintiffs who have experienced tooth decay from Suboxone films have filed more than 700 lawsuits as part of a multidistrict litigation. The decision on whether or not those lawsuits proceed will likely occur in 2025. There is a status conference scheduled for January 14, 2025, where a decision on the motion could be issued. Many in the legal community think these lawsuits will proceed to bellwether trials and eventual settlements for people harmed by Suboxone films.
December 18, 2024: Ruling Awaited On Motion to Dismiss Suboxone Tooth Decay Lawsuits
Many people are awaiting an important update on the ongoing Suboxone lawsuit. On December 16, 2024, the judge in the consolidated Suboxone lawsuits heard oral arguments on the defendant’s motion to dismiss. Judge J. Philip Calabrese is expected to rule on the motion in the coming weeks. Suboxone sublingual film’s manufacturer, Indivior, has requested for 716 lawsuits filed as part of a multidistrict litigation (MDL 3092) to be dismissed. Many legal experts think it is very unlikely this motion will be granted. After the oral arguments were presented, the judge issued an order confirming MDL hearing dates in 2025. The next MDL hearing will be held January 14, 2025.
December 12, 2024: Attorneys in Suboxone Lawsuit Prepare for Important December 16 Hearing on Lawsuit Dismissal
Attorneys on both sides of the Suboxone film lawsuits are preparing for an important hearing next week. The hearing will take place December 16, 2024, and discuss a Rule 12(b)(6) motion filed by the defendant. At this hearing, Judge Calabrese will decide if there are adequate grounds for Indivior to have the allegations and lawsuits against them dismissed. Indivior, who made Suboxone sublingual films, has asked for the lawsuits in the multidistrict litigation to be dismissed. Many people close to this case believe it is highly unlikely Indivior will be successful in this motion.
December 4, 2024: Court Will Hear Oral Arguments in Suboxone Lawsuits
On December 16, 2024, the judge in the Suboxone multidistrict lawsuit, J. Philip Calabrese, will hear oral arguments about a case-dismissal request from Indivior, the manufacturer of Suboxone sublingual films. This declaration was made in a November joint status conference. In July of 2024, Indivior filed a motion asking for the federal lawsuits against them to be dropped. Their reasoning for this request is they said federal law prevented them from updating the safety warning of Suboxone films, which have led to many users experiencing severe dental injuries. Judge Calabrese will rule on whether or not these lawsuits should be dismissed on these grounds. Many people in the legal community find this dismissal to be highly unlikely and assume the lawsuits will proceed.
December 2, 2024: More Cases Added to Federal Suboxone Lawsuits in November As People Come Forward With Dental Injuries
As of December 1, 2024, there are 716 lawsuits pending in the Suboxone multidistrict lawsuit (MDL). These lawsuits are part of MDL 3092, Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation. This means, since November 1, 2024, 38 more plaintiffs have joined the lawsuit after experiencing tooth decay, tooth loss, and other severe dental injuries. Settlements for these cases will likely occur after the bellwether trials are completed in 2025.
November 27, 2024: Suboxone Lawsuit Alleges Indivior Provided Financial Incentives To Doctors Who Prescribed Films Instead of Tablets
In a November lawsuit filed against Suboxone film manufacturer Indivior, a Kentucky man alleges that the company pressured doctors to prescribe Suboxone sublingual films as opposed to Suboxone tablets. The complaint filed by Douglas Rebholz said that Indivior “exerted pressure on the limited number of doctors authorized to prescribe Suboxone to create economic disincentives for doctors that did not transition their patients to the film from the tablet.” In doing this, the complaint alleges that Indivior put people who were using the Suboxone tablets at risk for severe dental problems by pushing physicians to instead prescribe them Suboxone film.
November 25, 2024: Plaintiffs Want Clean Process to Submit Medical Records for Suboxone Lawsuits
In a November 21, 2024, status conference for the Suboxone MDL, lawyers and Judge J. Philip Calabrese discussed the process for submitting medical records during the discovery phase (information-sharing phase) of the lawsuit. Lawyers want there to be a standardized process in order to expedite and facilitate the submission of these medical records. Judge Calabrese said that the lawyers should prepare a process and submit it to the courts for approval. If standardized, this new process would clearly outline how medical records should be submitted and what information those records should contain. This could help simplify case filings for plaintiffs.
November 22, 2024: Judge in Federal Suboxone Lawsuits Creates a Direct Case Filing Process
The judge in the Suboxone multidistrict litigation issued a direct filing process for claimants. In his November 22 Case Management Order, Judge J. Philip Calabrese stated, “any Plaintiff alleging dental injuries as a result of using Suboxone film may file her or his action against Defendants directly in this District as a member case of the MDL.” Attorneys representing people harmed by Suboxone films will follow this order to properly join their clients in this consolidated litigation.
November 21, 2024: Suboxone Films Found to Be Ten Times More Acidic Than Tomato Juice, Leading to Tooth Decay
As more people file lawsuits due to tooth damage associated with Suboxone sublingual strips, people are looking for answers as to why. Scientists have proposed that the acidity of the strips likely changes the microbial profile of the tooth, making that tooth more susceptible to damage. A large case series about Suboxone use published in the Primary Care Companion for CNS Disorders (PCC) found that Suboxone sublingual films had a pH of 3.4 when dissolved in water. This means Suboxone strips are ten times more acidic than tomato juices and sauces, which have approximate pH levels of 4.5. When patients held the strips against their teeth, they were likely altering the surface of their teeth in a negative way, leading to decay, caries, cracks, and additional dental conditions.
November 20, 2024: Kentucky Man Files Lawsuit After Experiencing Tooth Injuries Linked to Suboxone Use
Douglas Rebholz filed a lawsuit in Ohio federal court alleging his Suboxone use led to his severe dental injuries. He was prescribed Suboxone films for his opioid use disorder. After using the films, Rebholz started to experience severe dental issues. He now has permanent tooth damage that required substantial dental work to repair the damage. In his complaint, Rebholz’s attorneys say that he was never warned, either by prescribing information or his physicians, about the potential of serious dental side effects from Suboxone film use. In his complaint, Rebholz is pursuing compensatory damages, statutory damages, punitive damages, attorneys’ fees, and costs from Indivior.
November 19, 2024: Side Effect of Suboxone Films Has Been Tied to Dental Caries for Decades
Many people who used Suboxone sublingual films have lower saliva buffering capabilities. The negative effects of lower saliva buffering have been known for decades. In a paper published in the journal Dental Clinics of North America, dentists knew about the important role salvia buffering played in protecting teeth from caries, decay, infections, and other ailments. Scientific literature has been reporting on how low saliva buffering can harm teeth and gums. A comprehensive 2013 study said 90% of suboxone sublingual film users had low or moderate saliva buffering. This is another example of how Indivior should have known about the potential dental dangers associated with their Suboxone sublingual films.
November 18, 2024: Suboxone Film Users 67% More Likely To Experience Adverse Dental Events Like Tooth Decay
A data analysis published in the Journal of the American Medical Association (JAMA) found that people who used Suboxone films were 67% more likely to experience adverse dental events than people who used the oral tablet or transdermal form of the drug. The study evaluated data from 21,404 new users of sublingual buprenorphine/naloxone and compared their results with users of transdermal buprenorphine and oral naltrexone. This finding underscores the importance of proper dental care and follow-up for anyone who used Suboxone sublingual strips.
November 15, 2024: Joint Agenda Submitted For Case Management Conference in Suboxone Lawsuit
On November 15, 2024, the counsel in the consolidated federal Suboxone trial (MDL 3092) submitted an agenda for their next case management conference. The next conference will be held on November 21, 2024. The proposed agenda was submitted to Judge J. Philip Calabrese. The agenda for the conference includes: reviewing the status of an amended case management order, an update on the platform being used to store materials, the dismissal of a UK-based division of Indivior, and a status update on the production of adverse-event information.
November 11, 2024: Suboxone Lawsuit Judge Issues Order to Rectify Dual Representation for Some Plaintiffs
United States District Judge, J. Philip Calabrese, issued Case Management Order #12 in the consolidated Suboxone lawsuit (MDL 3092). In his November 4 order, Judge Calabrese set a timeline and expectations for plaintiffs who may have dual representation (i.e., plaintiffs who signed up for representation by two different attorneys). Some people who were harmed by Suboxone sublingual strips may have accidentally retained two different attorneys. The plaintiffs will have to choose a single lawyer to represent them. The attorneys who have clients flagged for dual representation have 21 days from November 4 to clarify which firm will represent each person. This order emphasizes the importance of plaintiffs registering with a single, reputable firm to represent them in their Suboxone lawsuits.
November 7, 2024: People Filing Suboxone Lawsuits Can Now Provide Evidence of Their Injuries to the Court
On November 4, the Judge in the Suboxone multidistrict litigation issued a case management order regarding the release of plaintiff health records to the court. Plaintiffs will provide Medical, Dental, and Pharmacy/MAT Authorizations to their dentists and other health providers. This will allow medical professionals to release medical records, imaging, and other evidence to the courts. This information will be used to support plaintiffs’ lawsuits and determine how much compensation each plaintiff might receive.
November 4, 2024: New Plaintiffs in Suboxone Lawsuit Prepare Information For Inclusion in Census Form
Each of the new plaintiffs who filed cases in October will need to supply a completed census form along with their case filing. The Suboxone Film Census Form has to be completed by each person who is seeking compensation for dental injuries linked to using Suboxone films. The census form contains information about a person’s specific injuries, the timeline of their suboxone use, and what type of damages they are suing for. Completing and filing this form is crucial to a successful lawsuit in the Suboxone multidistrict litigation. If this form is not filled and submitted properly, the plaintiff could be dismissed. Choosing a knowledgeable attorney is important for these cases.
November 1, 2024: More People Join the Federal Suboxone Lawsuit After Experiencing Dental Injuries
The federal suboxone sublingual film lawsuit (MDL 3092) continues to grow. As of November 1, there were 678 pending cases in the RE: Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation. This lawsuit is being overseen by U.S. District Judge J. Philip Calabrese in the Northern District of Ohio. We expect more plaintiffs will join this multidistrict litigation as people come forward after experiencing severe dental injuries from using Suboxone sublingual strips to treat their opiate use disorders.
October 28, 2024: Defendants Ready Information for November Status Conference for Suboxone Lawsuit
Ahead of the upcoming November 21, 2024, status conference, attorneys are preparing information for the federal Suboxone lawsuit. Indivior and other defendants who produced Suboxone sublingual strips have to present information about adverse events—events that harmed patients who took the drug. Reports presented by Indivior prior to the November status conference will contain information about the severe dental injuries experienced by people who used the sublingual strips. The Plaintiffs’ attorneys will be able to review this information ahead of the status call and use the call to discuss any concerns with the format or contents of the reports.
October 25, 2024: More Suboxone Lawsuits Filed in October as People Hold Indivior Accountable
As of October, there are 674 Suboxone lawsuits pending against Indivior, the Suboxone’s manufacturer. These cases are part of the multidistrict ligation (MDL 3092). The lawsuit against Indivior (IN RE: Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation) has been active for about 9 months. People who experienced severe dental injuries after using Suboxone strips are continuing to file lawsuits to gain compensation for their injuries.
October 21, 2024: Plaintiffs and Defendants Submit Legal Arguments in Federal Suboxone Lawsuit
Plaintiffs and defendants in the federal suboxone lawsuit (MDL 3092) continue to submit legal arguments to settle a Motion to Dismiss filed by the defendants. The defendants — including pharmaceutical companies Indivior and Aquestive Therapeutics — claim some plaintiffs cannot sue because the warning label was approved by the FDA when some of the plaintiffs used Suboxone to treat their opioid addiction. In response, the consumers argue the FDA’s approval of the drug’s label at certain times shouldn’t prevent them from filing a claim because Suboxone injured them. The court should decide on this issue in the coming months.
In this court document, the negligent organizations asked the court to partially dismiss two of the plaintiff’s arguments: The first is the plaintiff’s claim that Suboxone’s design is unreasonably risky for patients’ dental health. The second is that the defendants didn’t include enough information on the warning labels.
October 15, 2024: Status Conference Held in Federal Suboxone Lawsuit
From October 4, through October 5, 2024, a U.S. Federal Court in Ohio held an in-person status conference regarding the suboxone multidistrict litigation. Among the items discussed in this conference were the census protocol and census form. The census form is a questionnaire that each plaintiff fills out with personal information about their suboxone use, subsequent injuries, and treatments. Another item discussed was when the defendants will produce information about adverse events (e.g., dental injuries after taking suboxone strips). That data will be shared electronically before the next conference. The next suboxone status conference will be held November 21, 2024, at 9 a.m.
October 1, 2024: Lawyers to Submit Dental Injury Records as Court Prepares for Bellwether Case Selection
Lawyers in the Suboxone lawsuit were expected to submit information regarding dental injury records and product identification for cases on Monday, September 30th. The court is scheduled to meet again on Friday, October 4th, to discuss a final census form that will be used to select bellwether cases for trial. The trials will help evaluate how juries may respond to the evidence and could potentially influence future settlement amounts in the Suboxone lawsuit.
September 30, 2024: Kansas Resident Files Lawsuit Over Suboxone-Related Dental Issues
A Kansas resident filed a Suboxone lawsuit on September 23, 2024, stating he was prescribed Suboxone films by a physician to treat his opioid use disorder. There was no warning given of the dental erosion and tooth decay risk, which he now suffers from. He claims Suboxone has caused damage costing him thousands of dollars. The allegations against Indivior, the British manufacturer of Suboxone, include their knowingly and recklessly disregarding the rights and safety of consumers. He is asking for monetary damages for medical expenses and pain and suffering, including mental anguish, anxiety, discomfort, and more.
September 29, 2024: Rising Suboxone Prescriptions Before 2022 Warning Label Change May Fuel Lawsuits
Data from the National Institute on Drug Abuse (NIDA) shows that 2.5 million Americans aged 18 and older suffered from opioid addiction in 2021. With more people impacted by addiction, prescriptions for Suboxone increased as well. The proportion of people prescribed Suboxone rose from 3% in 2011 to 16% in 2020, and by 2022, over 16 million buprenorphine prescriptions were dispensed nationwide.
The increase in Suboxone use prior to the warning label change in June 2022 explains the growing number of claims in this lawsuit. As more individuals were prescribed Suboxone, the risks associated with its side effects, like tooth decay, have led to increased legal action against Indivior.
September 24, 2024: Suboxone Tooth Decay Lawsuit Progresses with Bellwether Trial Discussions
A status conference update was recently held in the Suboxone tooth decay lawsuit. The Court and the parties discussed the information that will be gathered from plaintiffs as the case progresses. Eventually, each person filing a lawsuit will be required to complete a detailed plaintiff fact sheet. The information gathered will be important for the next step in selecting candidates for creating a “bellwether pool.” Bellwether trials are “test” cases that are representative of the group of plaintiffs as a whole. They are used to evaluate discovery, evidence, and expert testimony, giving the parties an idea of how juries will react to the allegations. Typically, three to four trials are held out of thousands of cases. The results help drive settlement negotiations for the remaining plaintiffs. The next status conference is scheduled for October 4, 2024, with Judge Calabrese.
September 16, 2024: FDA Warning on Suboxone and Dental Problems Leads to Lawsuit Formation
The FDA foreshadowed the Suboxone lawsuit we are now seeing. In a press release posted on January 12, 2022, the FDA announced the requirement of a new warning about dental problems associated with the use of Buprenorphine. We believe that the 2022 warning is still inadequate. MDL 3092 In RE: Suboxone (Buprenorphine/Naloxone) Film Marketing, Sales Practices, and Products Liability Litigation was officially formed over two years later. The February 5, 2024 order consolidated 15 cases from five districts into a federal lawsuit centered in the Northern District of Ohio.
September 1, 2024: Judge Calabrese Sets Terms for Electronic Document Transfer in Suboxone Lawsuit
Judge Calabrese has established the terms for the transfer of electronic documents in the Suboxone lawsuit. Case Management Order No. 11, filed on August 28, 2024, outlines the timeline and definitions of what must be disclosed by the parties. Most discovery documents are produced by the defendants. This document production is a significant step in the lawsuit and is expected to take at least six months. The plaintiffs will then review millions of documents in search of evidence to support their claim that Suboxone films cause teeth to crack, break, or fall out.
August 25, 2024: Defense Files Partial Motion to Dismiss in Suboxone Lawsuit Over Dental Warning
The defense has filed a partial motion to dismiss one of the Suboxone lawsuits. The plaintiff responded on August 23, 2024. The central issue is whether the warning issued in 2022, which cautioned about potential dental problems, is sufficient. If deemed sufficient, claims for injuries that occurred after that time period could be dismissed. This warning also triggered the statute of limitations for thousands of potential Suboxone lawsuits. However, if the warning is found to be insufficient, it could significantly impact the entire Suboxone lawsuit. A ruling in favor of this plaintiff could strengthen the cases of other potential Suboxone plaintiffs, potentially leading to higher settlement amounts against Indivior.
August 18, 2024: Suboxone Lawsuit Strengthened by Evidence of Harmful Effects
We believe that the merits of the Suboxone lawsuit are strong. Several scientific studies show that Suboxone films are highly acidic, which can cause tooth decay. Indivior was aware of this but made Suboxone films anyway. Indivior also created a false narrative, alleging that the Suboxone pills, which were about to lose patent protection, were less safe than the films they were proposing. Suboxone films were a profit-driven move by Big Pharma. The U.S. Department of Justice caught on and arrested Shaun Thaxter, the Chief Executive Officer of Indivior, who was subsequently sentenced to federal prison. The drug has caused thousands of people to suffer from broken or lost teeth, resulting in permanent and severe injuries. This is the type of case that could lead to a quick settlement.
August 16, 2024: Key Legal Considerations for Suboxone Cases Prior to January 2018
Our lawyers are reviewing Suboxone cases for people who used the drug prior to January 2018. This date is significant because, in June 2018, generic Suboxone films became available, and most insurance companies only paid for the generic version. Any Suboxone use before this date involved the name-brand medication. The United States Supreme Court has held that the doctrine of preemption prevents individuals from suing generic drug manufacturers if the warning label matches that of the name-brand drug. This issue was decided in the case Pliva v. Mensing, which was determined by a 5-4 vote in 2011. To qualify for six months of use prior to the generic availability, a person must have started using Suboxone films by January 2018.
August 15, 2024: Washington Woman Files Lawsuit Over Dental Issues Linked to Suboxone Film
A Washington woman has sued pharmaceutical companies Indivior, Aquestive Therapeutics, and Reckitt Benckiser. The complaint, filed in the Northern District of Ohio on August 8, 2024, is part of the Suboxone Film Lawsuit. The plaintiff alleges that Suboxone films do not provide warnings about potential dental problems. She claims that after becoming addicted to opioids prescribed by a doctor, she was then prescribed Suboxone film to treat her opioid use disorder. However, she was not warned about the risk of tooth damage associated with Suboxone Film. As a result, she now suffers from tooth damage and has undergone significant dental repair work. The complaint further alleges that Indivior was aware of the risks associated with Suboxone films but chose to release them instead of the previously available pills, in order to maintain patent protection.
August 1, 2024: Billy Hensley v. Indivior Inc. in the Suboxone Lawsuit
A complaint filed on April 11, 2024, in the Suboxone Lawsuit provides insight into the allegations. Billy Hensley v. Indivior Inc. is document 1 in the federal court multidistrict litigation. The MDL is officially known as In re: Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation. The presiding judge is J. Philip Calabrese. The complaint spans 59 pages and alleges that Indivior, the manufacturer, knew or should have known that the acidity in the drug films would cause dental problems, including tooth loss, tooth decay, and broken teeth. It also claims that the defendants did not adequately warn users like Mr. Hensley of the risks. Billy Hensley is a resident of West Virginia, while Indivior is organized under the laws of Delaware and has its principal offices in Virginia. The case qualifies for federal court due to diversity, as the parties are from different states. The plaintiff alleges permanent and significant tooth damage.
July 26, 2024: Key Study Links Suboxone to Dental Issues, Prompting Lawsuits
The Suboxone lawsuit followed the release of an important study in the Journal of the American Medical Association. In December 2022, Mahyar Etminan, Ramin Rezaeianzadeh, and others published a paper titled “Association Between Sublingual Buprenorphine-Naloxone Exposure and Dental Disease.” Buprenorphine-Naloxone is the active ingredient in Suboxone strips. The study followed a random sample of buprenorphine users from 2006 to 2020. The study also found an increased risk of tooth loss or damage. The study suggested that the increased acid from oral Suboxone usage may be the cause of the problems. The Suboxone lawsuits began being filed in 2023, shortly after this journal article was published.
July 25, 2024: Suboxone Lawsuit Targets Indivior’s Pre-2018 Product Design Flaws
The Suboxone lawsuit is focused on Suboxone use prior to June 2018. In June 2018, generic Suboxone took over the vast majority of the market share. The Suboxone tooth decay lawsuit focuses on the defective design and production of brand-name Suboxone by Indivior. Indivior is the company that designed and tested Suboxone, and they knew or should have known of the risks. Indivior was formerly known as Reckitt Benckiser Pharmaceuticals Inc. The manufacturer already paid a $1.4 billion settlement in 2019 with the Department of Justice for illegally marketing Suboxone.
July 17, 2024: Judge Rules in Favor of Plaintiffs in Suboxone Lawsuit Discovery Dispute
There was an important ruling by Judge Calabrese in the Suboxone Lawsuit. The defense requested to split discovery into two phases. The first phase would be for determining whether Suboxone caused certain injuries, like tooth loss and broken teeth. Then, the only cases that would move onto phase two of discovery would be the cases where the plaintiff was successful in meeting their burden on general causation. The plaintiffs argued that this would cause undue delay. Judge Calabrese ruled in favor of the individuals who have allegedly been injured by Suboxone, allowing the case to proceed with all discovery. This decision is a win for the people claiming injuries due to Suboxone use.
July 11, 2024: Potential Tolling Agreement Signals Key Development in Suboxone Lawsuit
The most important development in the Suboxone lawsuit is the potential for a tolling agreement. Tolling agreements prevent plaintiffs from being required to file lawsuits within the statute of limitations. Defendants often argue that poor cases are filed into federal lawsuits. Plaintiff lawyers are often forced to rely on clients’ reported injuries because they have limited time to file lawsuits. Tolling agreements often come before settlements. Many lawyers think the Suboxone lawsuit could be lining up for an early settlement.
June 2024: As anticipated, there are now nearly 10,000 pending cases consolidated into the MDL. Individuals who began taking Suboxone prior to 2022 and suffered tooth decay, tooth loss, or other adverse dental issues are strongly encouraged to seek legal counsel immediately. There may only be a limited amount of time to take legal action due to state-specific statutes of limitation.
May 2024: The number of cases transferred to the MDL now totals over 200 and is expected to continue to increase due to the widespread use of opioid treatment.
March 2024: Dozens of cases are added to the MDL. Litigation is expected to grow into the thousands in the coming months.
February 2024: Multidistrict litigation (MDL) is approved in the Northern District of Ohio, consolidating dozens of similar cases alleging that the manufacturer of Suboxone failed to warn consumers about the potential for severe dental issues associated with the use of the medication.
November 2023: Suboxone manufacturer Indivior settles an antitrust class action lawsuit for $385 million. The antitrust lawsuit alleged that the company’s predecessor Reckitt Benckiser Pharmaceuticals, schemed to delay generic competition of the drug. In addition to this class action, the manufacturer reaches agreements on similar accusations with a number of other entities including $30 million for consumers.
September 2023: The first known lawsuit is filed against the maker of Suboxone regarding the medication’s potential to cause severe tooth decay.
June 2022: The label of Suboxone, which contains buprenorphine and is administered through a sublingual film, is updated to include a warning about potential adverse effects on the teeth and mouth, including severe tooth decay and tooth loss.
January 2022: The U.S. Food and Drug Administration (FDA) issues a warning about the risks of dental issues associated with the use of medications containing buprenorphine that are dissolved in the mouth.
About the Suboxone Lawsuit:
What is Suboxone and How Does It Work?
Suboxone Sublingual Film and Its Role in the Lawsuit
Suboxone Sublingual Film – Active and Inactive Ingredients
Suboxone Lawsuit Plaintiff Profiles
Suboxone Dental Injuries and Health Risks
Research Links Suboxone Use to Tooth Decay
FDA Issues Warning on Suboxone Use and Tooth Decays
Suboxone Manufacturer: Indivior Inc.
Deceptive Marketing Practices Regarding Safety of Suboxone
Who Qualifies to File a Suboxone Lawsuit?
Recoverable Damages in a Suboxone Lawsuit
How to File a Suboxone Lawsuit
Suboxone Lawsuit Statute of Limitations and Filing Deadlines
What is the Suboxone Lawsuit About?
The Suboxone lawsuit is based on thousands of claims that the medication increases a person’s risk of developing serious dental issues, including tooth erosion and decay. Manufactured by Indivior, Inc. (formerly known as Reckitt Benckiser Pharmaceuticals, Inc.), the drug was originally available in a dissolvable tablet before being switched to a sublingual film with a more acidic ingredient. Litigation alleges that the manufacturer switched to oral film to extend their monopoly and prevent generic manufacturers from being able to bring a lower-cost pill to market.
The film contains a 4:1 ratio of buprenorphine hydrochloride and naloxone hydrochloride dihydrate. Buprenorphine acts as a substitute for opioids and is known to help with withdrawal symptoms. In January 2022, the FDA issued warnings about the potential for buprenorphine to cause serious dental problems. The drug, in sublingual film form, had been on the market for ten years without any warning about the effects it could have on teeth or gums. In June 2022, a warning was finally added to the label.
Accusations against Indivior include that the company knew or should have known about the risk to the user’s dental health and failed to warn medical providers and consumers, putting profits over patients. Additionally, lawsuits allege that the company promoted the film as a safer, less abusable alternative to other opioid treatments. It is believed that the company obtained billions of dollars in revenue.
Is the Suboxone Lawsuit a Class Action or MDL?
While frequently used interchangeably, class action lawsuits and multidistrict litigation refer to two distinct types of legal actions. Class action lawsuits allow one or more plaintiffs to represent a larger group with similar claims. They can be filed in state or federal court. Multidistrict litigation (MDL) involves the consolidation of multiple lawsuits into one federal court to promote efficiency. Unlike a class action lawsuit, plaintiffs in an MDL maintain their individual status throughout the litigation.
Lawsuits have been filed nationwide against the manufacturer of Suboxone, alleging the medication causes severe dental issues. On February 2, 2024, the Judicial Panel on Multidistrict Litigation consolidated multiple lawsuits into an MDL for Suboxone lawsuits in an effort to streamline litigation.
The manufacturer of Suboxone, however, was previously the subject of a class action lawsuit involving the violation of antitrust laws. The litigation alleged that the pharmaceutical company strategized to prevent generic drug companies from offering a lower-priced version of the medication. The class action was eventually settled for a multi-million dollar payout.
What is Suboxone and How Does It Work?
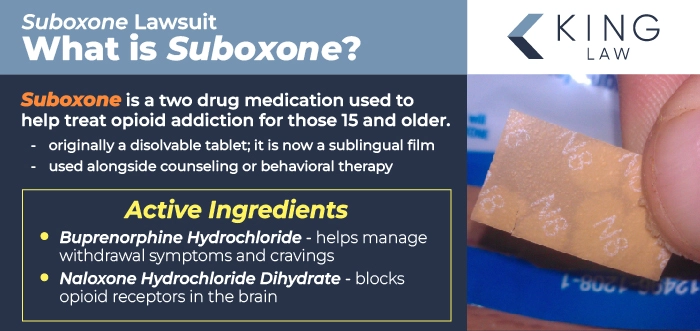
Suboxone is a two-drug medication used to treat opioid addiction in adults and adolescents over the age of 15. It is generally used as part of a comprehensive treatment program along with counseling. It is available by prescription and administered as a dissolvable film (sublingual or buccal), pill, or implant. The film tends to allow for easier dose tapering, but many find the tablets to be more discreet.
The medication combines buprenorphine, a partial opioid agonist, and naloxone, an opioid antagonist). It was frequently touted as a safer, less abusable opioid treatment that could help reduce withdrawal symptoms while preventing misuse-induced euphoria.
Suboxone mechanism of action and role in Medication-Assisted Treatment (MAT):
- Buprenorphine: Mimics opioid effects without euphoria and reduces withdrawal symptoms and cravings.
- Naloxone: Facilitates recovery by blocking opioid receptors to prevent misuse-induced euphoria.
Effects from Suboxone typically last from 24 to 36 hours, with common side effects being headache, nausea, constipation, and withdrawal symptoms.
Suboxone Sublingual Film and Its Role in the Lawsuit
Since 2012, Suboxone (buprenorphine and naloxone) has been prescribed in sublingual film to treat opioid addiction in adults. It is often considered an integral component of comprehensive treatment programs that include counseling and behavioral therapy. For maximum absorption, the sublingual film should be placed under the tongue and allowed to dissolve completely. Chewing the film too early may reduce its effectiveness or lead to withdrawal symptoms.
Prior to the introduction of the sublingual film, the drug had been prescribed as a dissolvable tablet. The film contains a much higher acidity (pH 3.4), which has been found to lead to enamel erosion, gum disease, and oral infections with prolonged use. Additionally, Suboxone users report experiencing reduced saliva production, which may exacerbate dental issues by reducing the mouth’s natural protective barrier against acidity.
In January 2022, the FDA issued a warning to consumers about the potential for adverse dental effects linked to buprenorphine after numerous complaints about the issue, including patients reporting the loss of teeth. Until this point, the medication did not contain any warnings related to the risk of enamel erosion, oral infections, or other dental problems. It is alleged that the manufacturer failed to adequately disclose the potential dental risks associated with the use of Suboxone.
Suboxone Sublingual Film – Active and Inactive Ingredients
Suboxone, an orange, rectangular soluble film, has a number of active and inactive ingredients. The ingredients, in combination with how the drug is administered, may cause serious side effects, including adverse dental issues.
Active ingredients in Suboxone:
- Buprenorphine Hydrochloride: Acts as a partial opioid agonist, helps manage opioid withdrawal symptoms and cravings, and mimics the effects of opioids without causing the same level of euphoria.
- Naloxone Hydrochloride Dihydrate: Acts as an opioid antagonist, blocks opioid receptors in the brain, reverses the effects of opioids, and can precipitate withdrawal if misused by injection.
Inactive ingredients in Suboxone:
- Acesulfame Potassium: Artificial sweetener used to improve taste.
- Citric Acid: Used as a flavoring agent and helps maintain acidity for stability.
- Maltitol: Sugar alcohol that is used as a sweetener, adding bulk to the formulation.
- Hypromellose: Polymer that is used as a thickening agent and enhances film formation.
- Polyethylene Oxide: Polymer that is used as a binder and aids in film consistency.
- Sodium Citrate: Used as a buffer and helps maintain pH balance.
- Natural Lime Flavor 3000180: Adds flavor to improve palatability.
- Sunset Yellow FCF: Synthetic food coloring that provides an orange color to the film.
Each film is imprinted with a logo indicating its dosage strength, i.e., “N2” (2 mg), “N4” (4mg), “N8” (8mg), and “N12” (12mg). Each pack is labeled with specific Australian Register Numbers (AUST R) corresponding to different strengths. It is recommended that the medication is stored in its original packaging at temperatures below 25°C to protect from light and moisture exposure.
Suboxone Dental Injuries and Health Risks
The use of Suboxone administered through a sublingual film has been linked to dental injuries and adverse health conditions, including tooth decay, oral infections, gum disease, and more. The medication’s high acidic pH is believed to exacerbate enamel erosion and increase a person’s susceptibility to tooth decay. Additionally, opioids are known to cause dry mouth or xerostomia, a condition that reduces saliva production, hindering the body’s natural ability to neutralize acid and repair enamel, as well as increasing the risk of dental caries.
According to a research letter published in the Journal of the American Medical Association (JAMA):
- Out of every 1,000 Suboxone users, approximately 21.6 will experience a dental adverse event each year.
- Out of every 1,000 Suboxone users, around 8.2 will develop dental caries or experience tooth loss each year.
Suboxone users may experience an increase in cravings for sugary foods and beverages, enhancing the risk for tooth decay, particularly when combined with dry mouth conditions. Because holding the film under the tongue is the most effective way to absorb it, users may have prolonged acidic exposure, further exacerbating enamel erosion. Finally, any normal neglect of oral hygiene resulting from lifestyle factors may raise the already present risk of dental decay.
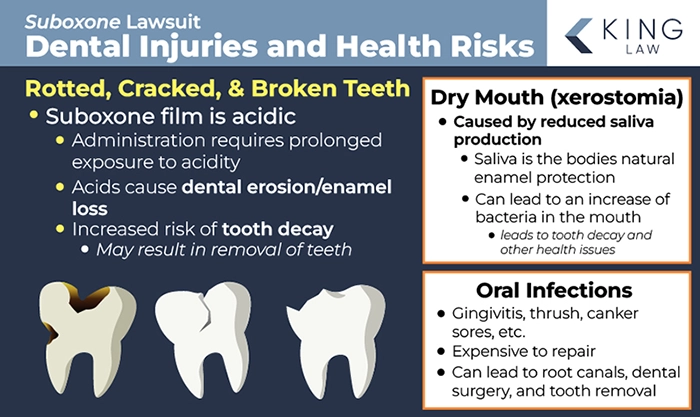
Rotted, Cracked, and Broken Teeth
One of the most prevalent adverse health conditions reported by Suboxone users is rotted, cracked, and broken teeth. The acidic nature of the sublingual film is believed to increase a person’s risk of tooth decay. Users are required to hold the film under the tongue until it is completely dissolved, generally taking several minutes and resulting in prolonged acidic exposure. Tragically, many users had to have permanent teeth removed as a result of the decay.
Oral Infections
Another common complaint of Suboxone users is repeated oral infections. Dental infections are costly to repair and can lead to additional problems, including root canals, dental surgery, and extraction.
Dry Mouth (Xerostomia)
The properties of Suboxone may also cause Dry Mouth or xerostomia. The condition causes a reduction in saliva in the mouth, reducing the body’s natural ability to protect the enamel of the teeth and counteract acidity. A lack of saliva can increase the amount of bacteria in the mouth, leading to tooth decay and other health conditions.
Other Dental Injuries and Health Issues
Suboxone patients have reported other dangerous dental injuries and health conditions such as gum disease, enamel erosion, and jaw problems. Treatment options for these issues are cost-prohibitive in many cases and may require extensive out-of-pocket payments. Common repairs include cavity fillings, crowns, crown replacements, and tooth extractions.
Research Links Suboxone Use to Tooth Decay
There have been several studies conducted on side effects related to Suboxone, particularly the potential for the medication to cause tooth decay and other dental conditions. As a result of over 300 reported adverse events, the FDA issued a warning to consumers about the risk of dental issues caused by buprenorphine. The warning, however, was issued a decade after the sublingual film was marketed to consumers. Prior to the FDA’s findings, the medication did not list any warning for the potential of serious dental side effects related to its use.
According to “Sublingual Buprenorphine and Dental Problems: A Case Series,” published in The Primary Care Companion for CNS Disorders, on average, each person had:
- Approximately 5 cavities, 4 fillings, and 2 cracked teeth.
- Needed 1 crown, 1 root canal, and 1 tooth pulled.
Over half (54.5%) of the people reported tooth pain, and 36.4% said they had low saliva buffering, meaning their saliva was less effective at neutralizing acid. Low saliva buffering is linked with a higher risk of cavities and other dental problems. Less than 10% had high saliva buffering.
The Research Letter published in JAMA, studied over 20,000 new users of sublingual buprenorphine/naloxone, 5,000 users of transdermal buprenorphine, and 6,600 users of oral naltrexone.
Their findings indicated:
- There was a higher incidence of dental problems with sublingual buprenorphine/naloxone (21.6 per 1000 person-years).
- There was a lower incidence with transdermal buprenorphine (12.2 per 1000 person-years) and oral naltrexone (10.9 per 1000 person-years).
- Sublingual buprenorphine/naloxone users have a 42% higher risk of dental issues compared to transdermal buprenorphine users.
- Sublingual buprenorphine/naloxone users have a 67% higher risk compared to oral naltrexone users.
- The incidence of dental caries was 8.2 per 1000 person-years for sublingual buprenorphine/naloxone users, 3.5 for transdermal buprenorphine users, and 3.8 for oral naltrexone users.
FDA Issues Warning on Suboxone Use and Tooth Decay
On January 12, 2022, after over 300 reports of adverse health reports received by users nationwide, the FDA issued a warning to consumers about the potential for an increased risk of dental problems associated with buprenorphine medications that dissolve in the mouth and are used for opioid use disorder.
Dental health issues were reported in some patients who had no prior history of dental problems. Of the 305 reported dental problems, 131 were classified as serious, with the average age of the patient being 42, although some patients were as young as 18. Dental problems began as early as 2 weeks after starting treatment, with a median diagnosis time of 2 years.
Issues reported to the FDA included:
- Tooth decay
- Cavities
- Oral infections
- Loss of teeth
Treatments for these conditions included tooth extraction/removal, root canals, dental surgery, crowns, and implants.
As a result of these reports, the FDA made several recommendations and requirements, including updated prescribing information, the addition of a warning about dental risks, and the inclusion of health maintenance strategies. The FDA recommended that individuals using buprenorphine medications engage in regular dental checkups and inform their providers about all medications they are taking. Additionally, preventative measures such as swishing water around after the medication has dissolved and waiting at least an hour before brushing are recommended.
Strategies recommended to healthcare providers included assessing the patient’s oral health history and referring patients to dental care services before prescribing the medication. During treatment, providers were suggested to counsel patients on potential dental risks and encourage regular checkups. Dentists should perform baseline dental evaluations and establish preventative plans for patients.

Suboxone Manufacturer: Indivior Inc.
Suboxone manufacturer Indivior, Inc. was founded in 1994 as a subset of Reckitt Benckiser but became independent in 2014. Its headquarters are in Richmond, Virginia. The company has expanded through strategic acquisitions such as Opiant Pharmaceuticals in November 2022.
Indivior focuses on buprenorphine-containing medications such as Suboxone tablets, which were introduced in 2002 for the treatment of opioid use disorder (OUD). Initially, the company faced little competition, transitioning to sublingual films when the trademarks on the tablets expired. It also manufactures Subutex, which only contains buprenorphine, and Sublocade, an extended-release injectable for OUD treatment.
It is believed that Indivior managed competition and maintained market control by moving to new formulations and introducing new delivery methods as their patents expired. The company settled multiple multi-million dollar lawsuits that alleged they violated antitrust laws related to these issues.
Deceptive Marketing Practices Regarding Safety of Suboxone
In addition to lawsuits related to the failure to warn consumers about the potential for adverse dental conditions related to the use of Suboxone, the manufacturer has faced legal action dating back to 2012 for its alleged deceptive marketing practices.
In July 2019, Indivior pled guilty to felony charges related to these claims and paid $600 million for criminal and civil liability. Individor’s predecessor, Reckitt Benckiser, resolved similar charges with a $1.4 billion settlement with the U.S. Department of Justice.
In October 2019, a $700 million settlement was reached with New York and five other states for improper marketing. These claims included that the drug was safer and less addictive than other products, false data was sent claiming the film had the lowest pediatric exposure rate, the promotion of the drug for non-medically accepted uses, and that it was less subject to abuse and diversion than similar medications.
Further allegations include that the manufacturer falsely claimed the tablets were discontinued out of safety concerns and that they strategically delayed the entry of generic competition to control Suboxone pricing.

Who Qualifies to File a Suboxone Lawsuit?
In order to file a Suboxone lawsuit, you must meet certain qualifications. To determine whether you meet the eligibility requirements, it is strongly recommended that you speak with an attorney as soon as possible.
Eligibility requirements for a Suboxone lawsuit include:
- You were prescribed Suboxone FILMS before January 2022.
- You used Suboxone for at least six months before injuries occurred.
- You experienced tooth loss or required tooth extractions, .
- You had routine dental care prior to taking Suboxone.
- You were not warned about the risk of dental injuries while taking Suboxone.
- You never used methamphetamine.
Additionally, your claim must be filed within the statute of limitations for product liability cases. It is strongly recommended that you consult with a qualified Suboxone tooth decay lawyer to determine if your claim is still valid and if an extension of time is possible.

Evidence to Gather Before Filing a Suboxone Lawsuit:
Prior to filing a Suboxone lawsuit, it is imperative to gather the necessary evidence to substantiate your case. Some of the most important things to consider and collect are medical records and dental records regarding your Suboxone use and subsequent injuries.
Evidence that may help to prove your case:
- Medical records
- Paperwork from your doctor and dentist appointments
- Proof of surgeries or procedures
- Any billing documents
- Dental x-rays and imaging reports
- Records of dental treatments
- Proof of Suboxone prescriptions
- Pharmacy records
- Detailed personal statements regarding dental issues
- Testimonies from family members or caregivers
- Insurance claims and reimbursements
- Records related to out-of-pocket expenses
- Statements from dental and medical experts linking Suboxone to dental issues
- Communications with healthcare providers discussing the risks and side effects of Suboxone
- Before and after photos showing the progression of dental damage
- Any documents that show a clear connection between Indivior’s negligence, your injuries, and your financial losses.
Recoverable Damages in a Suboxone Lawsuit
Individuals who meet the qualifications for a Suboxone lawsuit may be entitled to compensation. Recoverable damages may include economic and non-economic losses. To determine the value of your claim, you should speak with an attorney as soon as possible.
Economic damages in a Suboxone lawsuit may include recovery for:
- Medical bills: Costs of dental treatments, surgeries, procedures, follow-up care, and maintenance.
- Lost wages: Income lost due to time off from work for treatments and recovery.
- Loss of future earning potential.
Non-economic damages in a Suboxone lawsuit may include recovery for:
- Pain and suffering
- Physical and emotional distress
- Chronic pain, difficulty eating or speaking
- Embarrassment and social anxiety
Emotional distress includes the psychological impact of dental injuries, any anxiety, depression, and reduced quality of life experienced as a result of the dental conditions, lifestyle disruptions, and any impact on daily activities and social interactions.
How to File a Suboxone Lawsuit
To file a Suboxone lawsuit, you need to file certain steps that ensure your eligibility and that all legal requirements are met. It is important to understand that all Suboxone lawsuit cases are subject to a state-specific statute of limitations. An attorney can help ensure that your case is filed timely.
Step-by-step guide for filing a Suboxone lawsuit:
- Obtain a case evaluation: During your initial case evaluation, an attorney will help to ensure the viability of your case. Prior to your consultation, it is important to identify your symptoms and document evidence of your injuries, including all instances of tooth decay and any related dental issues. You should also gather medical records, dental records, and Suboxone prescription history.
- Legal preparation: The attorney you hire should be well-versed in product liability cases. They can help ensure that you collect the necessary evidence to help prove your case, including your full medical and dental history with all treatments and diagnoses related to your injuries. You will also want to secure any communications with your healthcare provider detailing Suboxone and its side effects. Finally, you will need to collect any receipts or bills for dental treatments related to tooth decay. An attorney can help you identify potential witnesses such as healthcare providers, family members, or friends who can attest to your Suboxone use and subsequent dental issues.
- Filing the lawsuit: Through your attorney, you will draft a complaint outlining the factors of your case and drawing the connection between your use of Suboxone and your tooth decay, as well as detailing the damages you are seeking. Your attorney will ensure that the complaint is filed in the appropriate court and that the document is formally served on all potential defendants, primarily the manufacturer of Suboxone.
- Discovery phase: Once the case is filed, it will enter into the discovery phase. The discovery phase allows for an exchange of information and documents between the parties. During this phase, there will likely be witness depositions and expert testimony.
- Pre-trial motions and settlement negotiations: Throughout the litigation process, each side may file pre-trial motions to try to resolve certain issues before trial. The motions may include attempts to dismiss the case or to compel the other side to produce evidence. The parties may also enter into settlement negotiations in an attempt to avoid going to trial.
- Trial: If a fair settlement cannot be reached, both sides prepare for trial. Trial preparation includes finalizing arguments, preparing witnesses, and organizing evidence. During the trial, the case is presented in court, and each side argues its case before a judge or jury.
- Post-trial: If you win the case, the court will determine the amount of damages to be awarded. If you lose, your lawyer may file an appeal if there are grounds to do so. If you win and the verdict is not appealed, the defendant will be ordered to pay the damages awarded by the court.

Suboxone Lawsuit Statute of Limitations and Filing Deadlines
All Suboxone lawsuits are subject to state-specific statutes of limitations, which are legal timelines within which cases must be filed. In general, the deadline for filing a Suboxone lawsuit with a two-year statute of limitations is June 14, 2024, due to the warning label changes that were made in June 2022. Plaintiffs’ lawyers were required to file a master MDL list by this date.
For Suboxone lawsuits with a three-year statute of limitations, the deadline for filing will be June 2025. It is not expected that many cases will settle prior to the passing of the three-year statute of limitations. The Discovery Rule, which can extend a statute of limitations beyond the standard two or three-year timeframe, applies from the time a person discovers their injury and knows it was caused by negligence.
Failure to file within the statute of limitations can result in you being barred from filing a lawsuit against Indivior or another defendant. A lawyer may assist in entering a tolling agreement, which can suspend the statute of limitations and protect your ability to file a claim. This helps to accommodate any delays that may occur in attempting to secure medical and dental records.
Suboxone Settlement and Payout Amounts
Suboxone settlement and payout amounts are expected to range between $50,000 and $150,000, depending on the individual circumstances of the case. Several factors may influence the value of a person’s case, including the severity of dental damage, treatment costs, and emotional suffering.
Factors that may influence the settlement value of a Suboxone claim:
- Past Medical Bills: Costs related to diagnosing and treating tooth decay, including dental visits, procedures, medications, and hospital stays.
- Future Medical Costs: Estimate of ongoing or anticipated treatments and care for tooth decay and related complications.
- Lost Income: Wages lost due to time off work for medical appointments and recovery.
- Future Earning Capacity: Impact on ability to work in the future, including potential career changes or diminished earning capacity.
- Physical Pain: Discomfort caused by tooth decay and related treatments.
- Emotional Distress: Psychological impact, including anxiety, depression, and loss of enjoyment of life.
- Disability: Long-term or permanent disabilities resulting from tooth decay and its treatment.
- Disfigurement: Permanent changes to appearance, such as missing teeth or dental implants.
- Loss of Consortium and Impact on Relationships: Effects on relationships with a spouse, children, and other family members, including loss of companionship, support, and intimacy.
- Punitive Damages and Manufacturer’s Conduct: Evaluation of whether the manufacturer acted with gross negligence, malice, or intentional misconduct.
- Comparative Fault and Shared Responsibility: Assessment of any shared fault in the case, compensation may be reduced proportionately if partially responsible for the condition.
Laws of the state where the lawsuit is filed can impact compensation, including caps on certain types of damages. Some plaintiff-friendly jurisdictions may result in higher compensation. It is important to know that the arrangement with your attorney is typically a percentage of the compensation awarded. Other legal costs include filing fees, expert witness fees, and court costs. To protect your settlement, you will want to practice good dental hygiene and attend dentist appointments. Be sure to address all dental emergencies promptly.
Contact a Suboxone Lawyer
If you were prescribed Suboxone prior to 2022 and suffered adverse dental issues, you may be entitled to compensation. It is imperative to contact a lawyer as soon as possible to determine whether you have a valid case. At King Law, we have extensive experience working with individuals in product liability cases, including those against drug manufacturers. Contact our office today to schedule a free, no-obligation consultation.



 Suboxone Tooth Decay Lawsuit: A Comprehensive Guide
Suboxone Tooth Decay Lawsuit: A Comprehensive Guide