
Rybelsus Lawsuit Overview
People are filing Rybelsus lawsuits after experiencing numerous severe side effects from taking the drug. Rybelsus, the brand name for an oral semaglutide manufactured by Novo Nordisk, has come under scrutiny after patients have reported serious side effects. Rybelsus, which originally received FDA approval in 2019 to treat type 2 diabetes, is commonly used for weight loss and acts as a GLP-1 receptor agonist.
It works by increasing insulin and decreasing blood sugar. The drug also causes delayed gastric emptying which can lead to adverse health issues, including gastroparesis (stomach paralysis) and bowel obstructions.
Lawsuits allege that the manufacturer failed to warn consumers about the potential for adverse health conditions associated with using the drug. Some health issues patients have reported are severe and can lead to long-term complications. Individuals who have suffered severe side effects after taking Rybelsus may be eligible to take legal action and should consult with an attorney as soon as possible.
Our firm is currently investigating Rybelsus lawsuits involving a diagnosis of:
- Gastroparesis/stomach paralysis
- Gastrointestinal Obstruction/ileus
- Daily vomiting lasting at least 3 weeks
- Vision loss/blindness/NAION
- Blood clots/deep vein thrombosis (DVT) or pulmonary embolism (PE)
If you have been diagnosed with any of the above conditions, or have experienced severe and prolonged vomiting, please contact us right away for a free Rybelsus lawsuit evaluation.
Rybelsus Lawsuit Update – 2025 Update
April 2, 2025: As Rybelsus Boasts Cardiovascular Benefits, Other Experience Severe Negative Side Effects
Data from a recent medical trial have demonstrated that Rybelsus cuts the risk of cardiovascular events by 14%. This news may have mixed reception to patients who are weighing the pros and cons of taking Rybelsus. Some people experience severe gastrointestinal injuries, vision loss, and blood clots while taking Rybelsus. Some of those people have pursued lawsuits to be compensated for their injuries. The total number of lawsuits against Novo Nordisk and other GLP-1 manufacturers topped 1,600 in March, and more people are expected to join this litigation as they sustain injuries from Rybelsus.
March 3, 2025: Lawsuits Involving Rybelsus Gain Momentum in 2025
Many people who experienced severe digestive injuries and other severe side effects after taking Rybelsus have joined a group lawsuit against the drug’s manufacturer, Novo Nordisk. There are now 1,521 active lawsuits in the group lawsuit against GLP-1 drug makers (the Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation). As more people harmed by Rybelsus file lawsuits, Judge Karen Spencer Marston is pressing the litigation forward. At a conference last week, she set a date for the Rule 702 hearing, which is a hearing where she will decide what information from which expert witnesses will be included in the trial. This hearing will take place on May 14, 2025. People harmed by Rybelsus still have time to file lawsuits and join this group litigation.
February 6, 2025: Lawsuits Against Rybelsus Manufacturer Novo Nordisk Increase
143 new lawsuits were filed related to injuries experienced after taking Rybelsus and other GLP-1 drugs. Between December 2024 and February 2025, 143 lawsuits were filed as part of the consolidated federal litigation against GLP-1 drug manufacturers. People continue to experience severe gastrointestinal injuries after taking Rybelsus, despite the fact that Novo Nordisk has not added warnings about gastroparesis, ileus, or similar injuries to the drug’s label. As people are injured by Rybelsus, they continue to join the consolidated lawsuit.
December 13, 2024: Warning Label for Rybelsus Updated to Include Gastrointestinal, Pancreatic, and Gallbladder Dangers
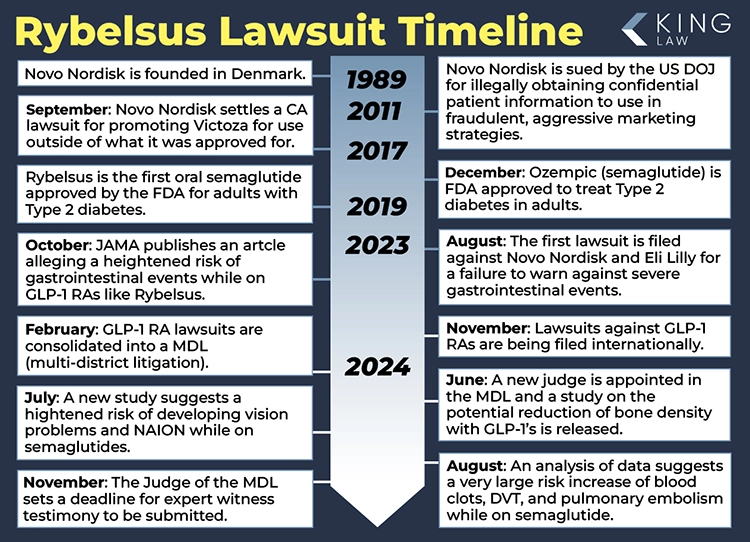
On December 9, 2024, the FDA posted an updated drug warning label for Rybelsus. The new label warns of acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. The FDA also added that patients who took Rybelsus were 2 to 7 times more likely to develop severe gastrointestinal issues than patients who took the placebo. The new label also warns of the risk of acute gallbladder information, called cholecystitis. These label updates reflect the severe side effects some people experience when taking GLP-1 drugs like Rybelsus. These injuries have led some patients to file lawsuits against Novo Nordisk.
December 2, 2024: 1,300 More People File Rybelsus and Other GLP-1 Lawsuits in November
The multidistrict litigation lawsuit (MDL) against Rybelsus manufacturer Novo Nordisk and other GLP-1 manufacturers has 1,300 pending lawsuits as of December 2, 2024. Many people who have filed lawsuits claiming that GLP-1 drugs caused them severe injuries have joined MDL 3094, Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation. As more people take GLP-1 drugs like Rybelsus to treat type-2 diabetes and lose weight, it is likely more people will join the MDL with the goal of receiving compensation and settlements.
November 22, 2024: Scientists Say More Research Is Needed on Rybelsus and Other GLP-1 Drugs and Possible Cancer Risks
A recent study examined data from people who took GLP-1 drugs like Rybelsus for 1 to 3 years. Researchers found those people had a 58% increased risk of developing cancers of the thyroid. The study, which was titled “Glucagon-like peptide 1 receptor agonists and thyroid cancer: is it the time to be concerned?” and published in Endocrine Connections, encouraged the scientific community to further examine GLP-1 drugs and whether they increase the risks of certain cancers.
November 7, 2024: Deadline for Submitting Expert Reports Set in Rybelsus Litigation
The judge in the GLP-1 multidistrict litigation (MDL 3094) has issued an order when attorneys for the plaintiffs and defendants have to submit expert reports. Judge Karen Spencer Martson gave plaintiff attorneys until November 18, 2024, to submit their reports. Defendants, including Rybelsus manufacturer Novo Nordisk, have until December 23, 2024, to submit their reports. Expert reports are written documents from expert witnesses that are used throughout different phases of the trial. These reports contain expert opinions and scientific evidence supporting those opinions.
October 4, 2024: High Number of Plaintiffs Join the Federal Rybelsus Lawsuit In September
Comparing numbers from August to September, there was a 20% increase in lawsuits filed against GLP-1 drug manufacturers as part of the consolidated federal lawsuit. As of October 1, 2024, there were 1,090 cases pending in MDL 3092, which includes Novo Nordisk, the maker of Rybelsus. This rapid increase in case filings is the result of more Americans taking drugs like Rybelsus and experiencing severe side effects. Tens of thousands of cases are expected to be filed against GLP-1 agonist drug manufacturers, including Novo Nordisk, in the coming months.
August 2, 2024: Comprehensive Data Analysis Suggests Rybelsus Linked to Blood Clots, DVT, and Pulmonary Embolisms
Trial data from an efficacy study point to an increased risk of blood clots for people taking semaglutide drugs, like Rybelsus, for type 2 diabetes. The findings were published in the Endocrine Journal. The article called attention to a 266% increase in the risk of deep vein thrombosis (DVT). This type of blood clot can cause pulmonary embolisms, which can lead to death. As more people take semaglutide drugs to treat their diabetes or achieve weight-loss goals, more people may be at risk for this potentially serious condition.
July 10, 2024: Rybelsus Lawsuit to Include Vision Loss Claims Following JAMA Study
A recent study in The Journal of the American Medical Association (JAMA) links the use of Rybelsus to a rare form of vision loss and blindness. Dr. Jimena Hathaway concluded that Rybelsus increases the risk of Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) by more than seven times in some patients. Dr. Hathaway reviewed over sixteen thousand cases involving semaglutide, the active ingredient in Rybelsus, Ozempic, and Wegovy. Semaglutide is manufactured by Novo Nordisk. Novo Nordisk has never warned of vision loss as a side effect of Rybelsus or other drugs using semaglutide. The Rybelsus lawsuit will now include vision loss cases. We are accepting clients who have used Rybelsus and experienced vision loss or blindness.
June 17, 2024: New Judicial Orders and Developments in MDL Proceedings
The first status conference in the Rybelsus lawsuit with the new judge, Karen Marston, was held on June 10, 2024. The focus was on the process of moving the MDL forward. Since the conference, the judge has issued two orders: first, lawyers who wish to file cases into the GLP-1 MDL will not have to pay a fee to be admitted pro hac vice; second, the parties have filed a motion, seemingly with permission from the judge, in regards to privilege logs. Parties in lawsuits are required to turn over relevant information to the other side. However, sometimes there are privileges that can be asserted. If a document is not turned over due to privilege, then the document is logged and the opposing side is informed that it exists.
June 8, 2024: New Judge Appointed to Rybelsus Lawsuit, In-Person Conference Scheduled
Case Management Order number 7 has informed the parties of a new judge in the Rybelsus lawsuit. Judge Karen Spencer Marston will now preside over this litigation. We expect the Ozempic lawsuit to be very large and include many complex legal motions. Judge Marston was a top student at Wake Forest Law School, and her legal acumen will certainly be tested in this case. The judge wasted no time in moving forward with the proceedings; an in-person conference is scheduled for June 10, 2024.
June 6, 2024: Rybelsus Lawsuit Paused Until New Judge Is Appointed Following Judge Pratter’s Death
The Rybelsus lawsuit in federal court is currently paused until a new judge is appointed due to the sudden death of Judge Pratter. We anticipate additional cases will continue to be filed in New Jersey state court. Federal court is the suitable venue when the involved parties are from different states. Cases against Novo Nordisk by New Jersey residents will likely need to be filed in New Jersey court. It is also possible that other plaintiffs will choose to file their claims in New Jersey state court.
May 2, 2024: Rybelsus Lawsuit Progresses with Scheduled ‘Science Day’ in June 2024
April 1, 2024: Key Developments in Rybelsus Lawsuit – Leadership Motion and New Jersey Filings
There are two important developments in the Rybelsus lawsuit. First, a group of lawyers has made an application to work together as the lead attorneys. The motion filed by plaintiffs’ lawyers seems like a compromise between several law firms. There is one objection to the leadership slate. We expect the group of nearly two dozen lawyers to be approved soon. We are pleased that our litigation partner, Dan Nigh, is a proposed member of the executive committee of the federal Rybelsus lawsuit. The second development is the filing of Rybelsus cases in New Jersey state court. Some lawyers feel it is an advantage to bring their cases away from the large group. The largest group of Rybelsus state court cases will likely be in New Jersey because it is the North American headquarters of Rybelsus manufacturer Novo Nordisk.
March 23, 2024: Leadership Committee Proposed in Rybelsus Lawsuit
Plaintiff lawyers have filed an important motion in the Rybelsus Lawsuit. The motion filed March 22, 2024, asks Judge Pratter to appoint several lawyers to leadership positions in the lawsuit against Rybelsus manufacturer Novo Nordisk. It is no surprise that our litigation partner Daniel Nigh, Esq. appears on the motion as an executive team member. Daniel is an experienced lawyer and has brought several cases against drug companies in the past, including a role as co-lead counsel for the valsartan lawsuit . Judicial appointment of lawyers to leadership roles is an important part of the case because it gives the lawyers the authority to start setting schedules for discovery and requesting documents from the manufacturer. We expect a large portion of the case to center around the marketing tactics used by Rybelsus manufacturer Novo Nordisk.
March 20, 2024: Rybelsus Lawsuit Status Conference Held
The first status conference in the Rybelsus lawsuit was held this last week. The Judge discussed how the plaintiff’s lawyers would work together to move the cases towards a bellwether trial. We expect an order on plaintiff leadership as soon as the end of the month. The judge also requested the parties participate in a Science Day. A science day will allow the lawyers to describe the scientific issues they expect to see in a case involving Rybelsus. Rybelsus is the same active ingredient as Ozempic and Wegovy, but different from Mounjaro. It is however the only drug that is taken in pill form, which will likely be addressed at science day and throughout the lawsuit. Finally, the lawyer for Eli Lilly requested an early motion for summary judgment date. The judge stated that the motion to dismiss was a long way away. There have been a lot of questions about whether Mounjaro and Eli Lilly should be part of the case. It appears the Judge thinks they are an appropriate party.
March 13, 2024: Novo Nordisk touts new weight loss pill
Novo Nordisk, the manufacturer of Rybelsus, has received a lot of press for a potential weight loss pill. Rybelsus was the first pill form of Semaglutide, a diabetes medicine that promotes weight loss. The drug was approved in 2019 and is typically taken in 7- or 14-milligram doses. Novo Nordisk confirmed they are testing a similar pill that would be 50 milligrams of Semaglutide and be approved for weight loss. Some indications are that higher doses lead to more severe side effects. This week Novo Nordisk also confirmed they have completed a Phase 1 trial of Amycretin, another pill designed specifically for weight loss. The Rybelsus lawsuit alleges that Novo Nordisk has not properly warned of the dangers associated with the use of the drug. The lawsuit also alleges that extreme marketing tactics have led to unreasonable risk of off-label use.
March 10, 2024: Key Date In Rybelsus lawsuit
This is an important week in the Rybelsus lawsuit. On Thursday there will be the first status conference before the Honorable Gene E.K. Pratter. We expect that several plaintiff’s lawyers will ask the judge to appoint them a leadership role in the case. The average Multi-District Litigation lasts three to five years, so it is a big commitment for the lawyers. We also expect there to be millions of documents produced related to the design, testing, and adverse event reports around the drugs included in the lawsuit. The burden of proof is on the plaintiffs and it is a large undertaking. One of the first important issues to address will be what drugs will be part of the lawsuit. We expect that at a minimum Rybelsus, Wegovy, and Ozempic will be included because they have the same active ingredient Semaglutide, which is patented by Novo Nordisk.
March 2024: There are currently 58 lawsuits filed against Novo Nordisk, the manufacturer of Ozempic, Rybelsus, and Wegovy.
February 2024: The U.S. Judicial Panel on Multidistrict Litigation ordered all federal Ozempic lawsuits to be centralized in the Eastern District of Pennsylvania. Ozempic is an injectable semaglutide manufactured by the same company as Rybelsus.
September 2023: The U.S. Food and Drug Administration requires Novo Nordisk to add warnings to Ozempic that the semaglutide may cause serious gastrointestinal issues such as intestinal blockage and ileus.
January 2023: The U.S. Food and Drug Administration approves a label update for Rybelsus tablets. The update allows Rybelsus to be used as a first-line treatment for type 2 diabetes, removing a previous requirement that it not be the initial therapy for patients with the condition.
On this page:
Rybelsus Side Effects and Symptoms
Rybelsus FDA-Approval and Its Black Box Warning
Manufacturer of Rybelsus – Novo Nordisk
Rybelsus and Its Ingredient Breakdown
Eligibility for Filing a Rybelsus Lawsuit
Statute of Limitations for Filing a Rybelsus Lawsuit
How to File a Rybelsus Lawsuit
What Is Rybelsus?
Rybelsus was the first glucagon-like peptide (GLP-1) receptor protein oral treatment approved in the United States. It is a semaglutide like Ozempic and Wegovy, but does not need to be injected. The drug was approved by the U.S. Food and Drug Administration (FDA) on September 20, 2019, to help treat type 2 diabetes in adults. It is a non-insulin treatment taken in 7-mg or 14-mg once-daily oral doses.
The drug, manufactured by Novo Nordisk, helps to improve blood sugar levels and has been known to promote weight loss by regulating appetite and slowing digestion. It mimics the GLP-1 hormone causing a delay in gastric emptying resulting in patients feeling fuller longer.
Reports have emerged showing a link between the drug and serious side effects such as gastroparesis and bowel obstruction. Lawsuits allege that it has a higher risk of gastrointestinal issues compared to other weight loss drugs. Accusations against the manufacturer include that they knew of the increased risk for adverse health issues, especially with long-term use, and failed to warn consumers.

How Does Rybelsus Work?
Rybelsus works as a glucagon-like peptide (GLP-1) receptor antagonist. It mimics a hormone that regulates a person’s blood sugar and their appetite. The drug enhances insulin production after a meal and reduces glucagon secretion which lessens glucose production by the liver. Patients report a longer feeling of fullness since the oral tablet helps to slow digestion.
While the primary use of Rybelsus is to treat type 2 diabetes by preventing the liver from making too much sugar and helping the pancreas to produce more insulin, many people use it to help promote weight loss. The drug signals the brain to regulate appetite, which can lead to a reduction in calorie intake.
It can have a significant impact on the digestive system since it delays gastric emptying which may affect the absorption of other oral medications. Despite patient reports of serious gastrointestinal issues after use, the label does not give a specific warning for gastroparesis or stomach paralysis.
Rybelsus Side Effects and Symptoms
Concerning reports have emerged alleging severe side effects after the use of Rybelsus and other semaglutide drugs. Lawsuits allege that the manufacturer of Rybelsus, Novo Nordisk, knew of these side effects and failed to inform consumers. Side effects associated with use of the drug can cause long-term health complications that can have a personal and financial impact on patients.
Reported side effects and symptoms associated with the use of Rybelsus:
- Gastroparesis or stomach paralysis
- Bowel and intestinal obstruction
- Deep vein thrombosis (DVT) and pulmonary embolism (PE)
- Severe, chronic vomiting
- Injury to the esophagus
- Ileus or intestinal blockage
- Inoperative pulmonary aspiration
- Pancreatitis
- Pancreatic cancer
- Serious allergic reactions
- Thyroid tumors, including cancer
- Disorders related to malnutrition
- Hypoglycemia or low blood sugar
- Kidney failure
- Vision changes / vision loss / blindness (NAION)
Individuals who suffered these or other side effects after using Rybelsus may be able to take legal action against the manufacturer for a failure to adequately warn consumers of the adverse effects of using the drug. It is believed that there is an increased risk of severe medical conditions associated with the long-term use of Rybelsus.
Most Common Side Effects of Rybelsus
While there are wide-ranging reports of dangerous side effects linked to the use of Rybelsus, some health risks are more common than others. The severity, however, varies from mild gastrointestinal issues to severe health concerns such as gastroparesis and the potential to develop thyroid tumors.
Common side effects associated with Rybelsus:
- Constipation and diarrhea
- Stomach or abdominal pain
- Nausea and vomiting
- Decreased appetite
- Heartburn
- Gas and bloating
- Flu-like symptoms
- Gallbladder issues
When side effects are undisclosed or patients are not made aware of the potential for adverse health complications, doctors and consumers are unable to make informed decisions.
Blood Clots/DVT and Rybelsus
As a semaglutide drug, Rybelsus may put people at risk of developing a specific type of blood clot, called deep vein thrombosis (DVT). This possible side effect was highlighted by an analysis of data from the PIONEER and SUSTAIN efficacy trials. The findings were published in the Endocrine Journal.
The comprehensive analysis found a 266% increase in DVT for people taking semaglutide drugs to treat their type 2 diabetes. DVT is a condition where a blood clot forms in a deep vein within the body, often in a leg. These clots can lead to serious complications.
Pulmonary Embolisms and Rybelsus
Rybelsus can lead to DVT, and these clots can lead to a serious event called a pulmonary embolism (PE). A pulmonary embolism occurs when a clot breaks free and travels to the lungs. Once the clot lodges in the blood vessels of the lungs, it can cause lung damage or block circulation, which can lead to death. More than 100,000 deaths each year are attributed to PEs caused by DVT.
As more people take semaglutide drugs, like Rybelsus, for management of their diabetes or for weight loss, increasing numbers of people may be at risk for this serious complication.
Vision Problems and Rybelsus
A recent medical study suggested semaglutide drugs, like Rybelsus, may cause vision problems. These vision problems are caused by non-arteritic ischemic optic neuropathy (NAION). NAION occurs when the optic nerve does not get enough blood flow. This can cause an eye stroke and vision problems. These vision issues are likely permanent and untreatable.
The July 2024 study was published in JAMA Ophthalmology and examined people taking semaglutide drugs for diabetes treatment or weight loss. The study found people taking semaglutide drugs were about three times more likely to develop NAION.
People who take Rybelsus may be at an increased risk of vision problems due to the drug. If you or someone you love took Rybelsus and experienced vision loss, please contact our team to discuss your case and possible compensation.
Rybelsus FDA-Approval and Its Black Box Warning
On September 20, 2019, the FDA approved Rybelsus as the first oral GLP-1 treatment for type 2 diabetes. Prior to that date, only injectable GLP-1 treatments had been approved such as Ozempic. While it is currently used off-label for weight loss, it is not approved for this use through the FDA.
In January 2023, Novo Nordisk announced that the FDA had approved a label update for the drug. The updated label allows Rybelsus to be used as a first-line treatment option for adults with type 2 diabetes. Prior to this date, it was not approved for use as the initial therapy for patients with the condition.
Rybelsus Black Box Warning
Due to the potential of serious adverse reactions when using the drug, Rybelsus is required to carry a black box warning. Black box warnings are required by the FDA when there is a serious risk of harm to patients.
As indicated by the label, studies on lab animals showed an increased risk in the development of thyroid cancer, including cancer. Patients with a family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2,0 (MEN 2) are at higher risk of thyroid C-cell tumors and should not use Rybelsus.
Manufacturer of Rybelsus – Novo Nordisk
Novo Nordisk is the manufacturer of Rybelsus, Ozempic, and Wegovy. Semaglutide, the generic name for the drug, was tested in clinical trials from 2016 to 2017, prior to approval by the FDA to treat type 2 diabetes in adults. Ozempic, an injectable semaglutide was approved in 2017, while Rybelsus, an oral version of the drug was approved in 2019.
Wegovy, an injectable semaglutide, is the only drug of the three approved by the FDA for weight loss. Rybelsus is in clinical trials to determine if it is a safe and effective weight loss treatment. All three drugs have faced scrutiny after numerous patients reported serious side effects with their use. Nationwide lawsuits allege that Novo Nordisk knew of the gastrointestinal risks associated with the use of semaglutide and failed to warn consumers.
Rybelsus and Its Ingredient Breakdown
The active ingredient in Rybelsus is 14 mg of semaglutide, produced using DNA technology. However, there are a number of inactive ingredients in the oral tablet that are kept in pill form and stored in a bottle.
Inactive ingredients in Rybelsus:
- Magnesium stearate
- Microcrystalline cellulose
- Povidone
- Salcaprozate sodium – 23 mg
The active ingredient in Rybelsus has been linked to a number of severe health risks. Individuals who have experienced adverse health conditions after using Rybelsus or another semaglutide are strongly encouraged to consult with a legal representative.
Semaglutide Usage Linked to Severe Health Risks
There have been several serious health risks linked to the use of semaglutide, the generic name for Rybelsus. These health risks include stomach paralysis and bowel obstruction. Prolonged use of the medication may increase the likelihood that a patient will develop one or more of these conditions.
Semaglutide is used to regulate blood sugar levels and increase insulin secretion; however, it may also provide a longer feeling of fullness aiding in weight loss. Unfortunately, the drug is usually prescribed in a higher dose when used for weight loss, increasing the risk that a patient will develop severe side effects such as gastroparesis, malnutrition-related disorders, deep vein thrombosis, and bowel obstruction.
Eligibility for Filing a Rybelsus Lawsuit
In order to file a Rybelsus lawsuit, you must first meet certain criteria, including proof of use of the drug. Working with an attorney can help you determine whether you may qualify for damages.
Eligibility criteria for a Rybelsus lawsuit:
- You must be able to show that you were actively using Rybelsus.
- You suffered adverse health conditions including ileus, gastroparesis, gastro-intestinal blockage, bile duct cancer, Lou Gehrig’s Disease (ALS), gallbladder cancer, sinus cancer, vision loss, DVT, PE, or another serious side effect after taking Rybelsus.
- You have evidence from a doctor or gastroenterologist linking your condition to the use of Rybelsus.
- You used the drug for weight loss purposes.
- You required medical intervention as a result of using the drug such as hospitalization, emergency room visits, or a consultation with a gastroenterologist, ophthalmologist, or other medical professional.
The best way to determine if you meet the criteria for filing a Rybelsus lawsuit is to contact an attorney. It is important to note that you only have a limited amount of time to take legal action.

Evidence to Collect Before Filing Your Lawsuit:
If you have experienced significant health problems after taking Rybelsus, you may qualify for a personal injury lawsuit. Eligibility may depend on a number of factors including your ability to prove how long you were taking Rybelsus and the injuries you experienced.
Evidence that may strengthen your Rybelsus lawsuit:
- Medical records
- Proof of prescription
- Emergency room visits and hospitalizations
- Doctor’s notes
- Documentation of missed work
The more evidence you can collect, the stronger your case will be. It is important to consult an attorney as early in the process as possible to determine your legal options.
Statute of Limitations for Filing a Rybelsus Lawsuit
All personal injury lawsuits are restricted by a statute of limitations, or legal time limit. Statutes of limitations are state-specific. An attorney can help determine if there are any exclusions or exceptions to the statute of limitations that may apply to your case. In many instances, the statute of limitations ranges between one and three years.
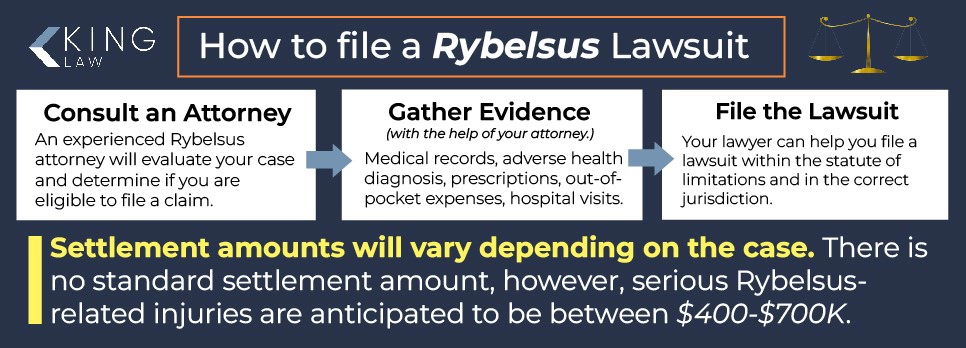
How to File a Rybelsus Lawsuit
You will need to follow several steps before filing a Rybelsus lawsuit. A lawyer can help you navigate the process and ensure you receive the best possible outcome in your case.
Steps for filing a Rybelsus lawsuit:
- Determine eligibility: In order to determine eligibility you should contact a lawyer for a free case review. During the initial consultation, an attorney will help determine if you have a valid claim for damages.
- Gather evidence: The second step in a Rybelsus lawsuit is evidence gathering. In order to strengthen your case, you will need to collect evidence such as medical records, proof of out-of-pocket expenses, prescription documents, and expert testimonies.
- Filing the lawsuit: An attorney can aid you in filing the lawsuit in the correct jurisdiction and within the statute of limitations.
- Negotiations: Your attorney may enter into negotiations with the defendant to secure a favorable settlement in your case. If a settlement cannot be reached, your attorney may set the case for trial to obtain the outcome you deserve.
Throughout the proceedings, it is strongly recommended that you work with a qualified Rybelsus lawsuit attorney.
Rybelsus Settlement Amounts
The anticipated settlement range for serious injuries related to a Rybelsus lawsuit is between $400,000 and $700,000; however, the amount will vary greatly depending on the case. Since there is no standardized settlement amount, the value of your case will be determined by your individual damages.
Damages generally reflect the extent of your losses and the severity of your injuries. An attorney can help ensure that you receive the maximum compensation allowed by state or federal law.
Contact a Rybelsus Lawyer to File a Lawsuit
If you have suffered injuries related to your use of Rybelsus, it is strongly recommended that you contact a qualified attorney. At King Law, our legal team has decades of combined experience handling prescription drug claims. We are well-versed in handling complex lawsuits and will help you secure the compensation you deserve. Contact our office today to schedule a free, no-obligation consultation.
Frequently Asked Questions (FAQs)
Find answers to any major questions you have regarding eligibility in the Rybelsus lawsuits below.

