While Ozempic has shown promising results for the treatment of diabetes and weight management, it has also gained attention for severe side effects. Stomach paralysis and intestinal blockages have been large concerns for Ozempic patients. And now, blindness is a concern. New findings connect semaglutide medications like Ozempic, Wegovy, and Rybelsus to a rare eye condition that causes sudden, often irreversible, blindness in one eye. As a result, some are pursuing legal action through Ozempic blindness lawsuits.
Ozempic Blindness Lawsuit Overview
Ozempic blindness lawsuits claim that the drug’s manufacturer, Novo Nordisk, failed to adequately warn consumers about the risk of blindness caused by nonarteritic anterior ischemic optic neuropathy (NAION). There are several other eye issues that may warrant legal action, also linked to semaglutide medications like Ozempic. These lawsuits can highlight the impact of blindness and other eye injuries on the consumer’s mental health, ability to work, financial stability, and more. Ozempic lawsuits can hold Novo Nordisk accountable for any wrongdoings and provide plaintiffs with compensation to help with medical bills and other damages.
Ozempic Blindness Lawsuit Updates
Stay up to date on the latest litigation for Ozempic and blindness.
September 16, 2024: Blindness and Vision Loss Becoming A Growing Concern in the Ozempic Lawsuits
The American Association of Ophthalmology suggests that Ozempic and other GLP-1 drugs can cause blindness or vision loss in several ways. The most common vision problem associated with Ozempic and other semaglutides is diabetic retinopathy. Other, more sudden changes in vision, like eye strokes and NAION, can lead to blindness, particularly in one eye. We expect future studies on vision loss associated with Ozempic use to be an important part of the Ozempic vision loss lawsuits.
July 14, 2024: Ozempic Lawsuit Intensifies with New Studies Linking Drug to Vision Loss
The Ozempic blindness and vision loss lawsuit is full steam ahead following the July 2024 study indicating huge increases in the risk of NAION for users of semaglutide. Another study worth considering is the 2020 study that found over 140 adverse vision effects from the use of semaglutide, the active ingredient in Ozempic. The study was published in Investigative Ophthalmology and Visual Science in June 2020. This study closely assessed the results of the Ozempic clinical trial and was also relied on for a previous lawsuit involving a Northeast Ohio woman who experienced sudden blurred vision after her third dose of Ozempic. We expect an application to include vision loss cases in the federal lawsuit shortly.
July 2024: New Study Connects Ozempic to Blindness
Blindness has emerged as a prominent concern for Ozempic patients following new findings that link semaglutide to sudden, often permanent vision loss in one eye. This study has raised questions about why Novo Nordisk has not warned patients about such serious side effects, similar to concerns regarding stomach paralysis when taking the medication.
January 2024: Researchers Examine Eye Risks Associated with Ozempic
A systematic review of patients taking semaglutide revealed multiple primary and secondary outcomes. Notably, blurred vision, retinopathy, and other macular complications were identified as exploratory outcomes, indicating the need for further research to fully understand all the risks associated with this medication.
August 2021: Ozempic Could Be Linked to Increased Risk of Diabetic Retinopathy
Study results examining diabetic retinopathy (DR) in patients taking semaglutide found “a higher risk for DR complications in patients with proliferative and non-proliferative DR at baseline.” However, researchers acknowledge that worsening DR could be due to rapid drops in blood glucose levels from diabetic medications.
June 2020: Initial Findings Connect Ozempic to Diabetic Retinopathy and Other Ocular Issues
A study found that Ozempic is linked to diabetic retinopathy and adverse ocular events more frequently than other GLP-1 receptor agonists. Reported cases of diabetic retinopathy and adverse ocular events for Ozempic were 16.4% and 1.1%, respectively. In comparison, other diabetic medications showed a range of 2.3%-6.3% for diabetic retinopathy and 0.05%-0.19% for adverse ocular events.
About the Ozempic Blindness Lawsuit:
What Is Blindness From Ozempic?
How Ozempic Causes Blindness and Vision Problems
New Study Links Ozempic and Rare Eye Disease NAION
Percentage of Ozempic Users Reporting Blindness
Ozempic Manufacturer’s Response to NAION Allegations
How to File an Ozempic Blindness Lawsuit
What Is Blindness From Ozempic?
A recent study conducted by the Harvard teaching hospital Mass Eye and Ear found an increased risk of developing nonarteritic anterior ischemic optic neuropathy (NAION) in individuals taking Ozempic. NAION is an eye condition caused by decreased blood supply to the optic nerve. It causes sudden, painless vision loss in one eye with irreparable damage to the nerve and impacts the eye’s connection to the brain. Often referred to as an “eye stroke,” NAION doesn’t have a known treatment option and can be permanent.
NAION occurs at an estimated rate of 2.3-10.2 per 100,000 people in the United States above the age of 50. However, it may become more prevalent with the popularity of Ozempic. According to the Mass Eye and Ear study:
- 8.9% of diabetic patients taking semaglutide received a NAION diagnosis.
- 1.8% of diabetic patients taking other medications received a NAION diagnosis.
- 6.7% of overweight or obese patients taking semaglutide received a NAION diagnosis.
- 0.8% of overweight or obese patients taking other medications received a NAION diagnosis.
- Patients with diabetes who took semaglutide were more than four times more likely to develop NAION than those on other medications.
- Patients who were obese and took semaglutide were more than eight times more likely to develop NAION than those on other medications.
Because of how serious NAION is, doctors are encouraged to let their patients know that it’s a potential side effect of Ozempic. Patients should also monitor closely for any potential eye symptoms and seek medical care immediately should they notice anything abnormal. While more research is needed to further understand NAION and Ozempic, the results are strong enough to warrant action and attention.
How Ozempic Causes Blindness and Vision Problems
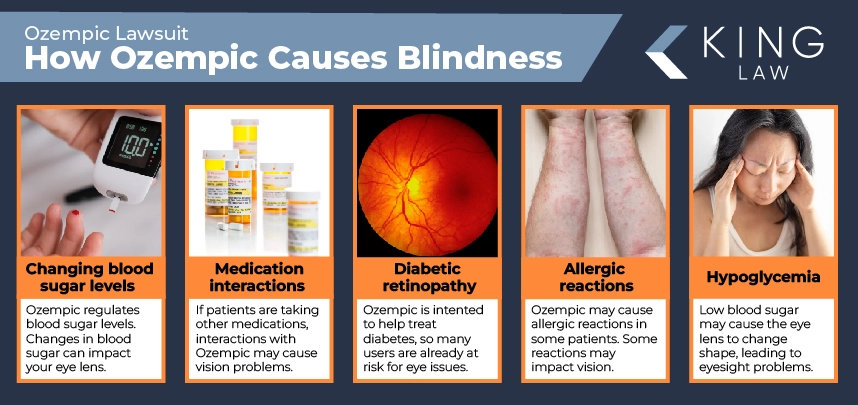
Additional research is needed to confirm and expand upon the Mass Eye and Ear study. Right now, it’s unclear how exactly Ozempic causes blindness and vision problems. Potential causes include:
- Changing blood sugar levels: Ozempic helps regulate blood sugar. Changes in blood sugar can impact your eye lens, causing blurry vision and damage to blood vessels in the retina.
- Diabetic retinopathy: Ozempic is intended to help treat diabetes, so many Ozempic users are already at a heightened risk for some eye conditions, including diabetic retinopathy, which impacts vision.
- Hypoglycemia: Ozempic can help patients with blood sugar issues. If blood sugar is low (hypoglycemia), the eye lens may change shape, leading to blurry vision and other problems with eyesight.
- Medication interactions: If Ozempic patients are taking other medications, which can be coming for diabetes, vision issues could be a side effect of drug interactions.
- Allergic reactions: Ozempic may cause an allergic reaction in some patients. Depending on the type of reaction, vision may be impacted.
To help prevent or treat these issues, patients should let their healthcare provider know that they are taking Ozempic. They should also maintain regular eye examples and monitor blood sugar to identify large fluctuations. Patients should seek medical care as soon as possible if they experience any adverse reactions to Ozempic.
Ozempic Eye Side Effects
Closely managing diabetes and monitoring eye health can help healthcare providers identify any conditions early on. Patients may need to discontinue Ozempic or seek additional treatments. Here is a comprehensive list of potential eye-related side effects for diabetic patients taking Ozempic:
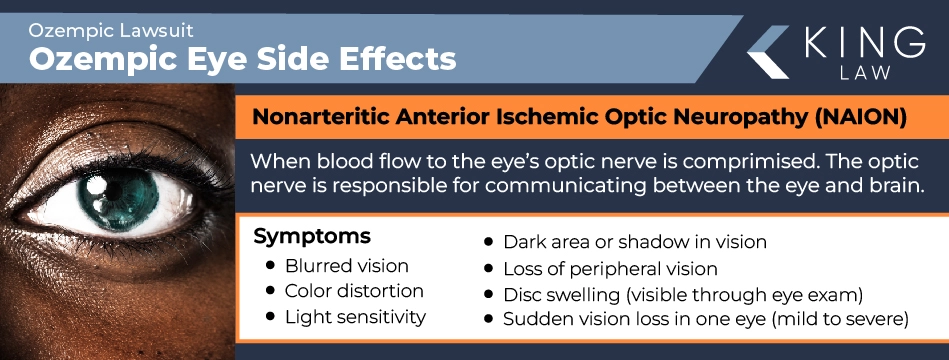
Nonarteritic Anterior Ischemic Optic Neuropathy (NAION)
Nonarteritic anterior ischemic optic neuropathy (NAION) is caused by compromised blood flow to the eye’s optic nerve. This can cause permanent damage to the front of the optic nerve (ischemia), which is responsible for your vision as it communicates between the eye and the brain. Symptoms of NAION can include:
- Sudden, mild to severe vision loss in one eye
- Blurred vision
- Color distortion
- Light sensitivity
- Dark area or shadow in vision
- Loss of peripheral vision
- Disc swelling (visible through eye exam)
Potential risk factors of NAION include:
- Older than 50 years old
- Diabetes
- Hypertension
- High cholesterol
- Sleep apnea
- Some anatomical predispositions
- Medications like semaglutide (Ozempic) and sildenafil (Viagra)
Because Ozempic is intended to help manage blood sugar levels in diabetic patients, changing blood sugar can lead to eye damage and vision changes.
Eye Floaters
Eye floaters are when individuals see abnormal spots in their vision. They’re usually small and dark, and can look like spots, lines, cobwebs, or other shapes. Floaters are most noticeable when looking at a bright, simple background that provides contrast. Potential causes of eye floaters include:
- Age-related changes: Vitreous shrinkage where material inside the eye shrinks, becomes more liquid-like, and can cause clumps or strands within the eye that cast shadows on the retina.
- Eye inflammation: Infection, injury, or autoimmune responses can cause inflammation of the uvea (uveitis).
- Eye bleeding: Diabetes, hypertension, or injury can cause bleeding inside the eye.
- Torn retina: Aging, injury, or retina thinning can cause the retina to tear or detach.
- Eye medications: Some medications are injected into the vitreous to help treat retinal conditions.
Ozempic may also contribute to eye floaters through the various scenarios listed above including, diabetic retinopathy, blood sugar fluctuations, hypoglycemia, inflammatory responses, and drug interactions.
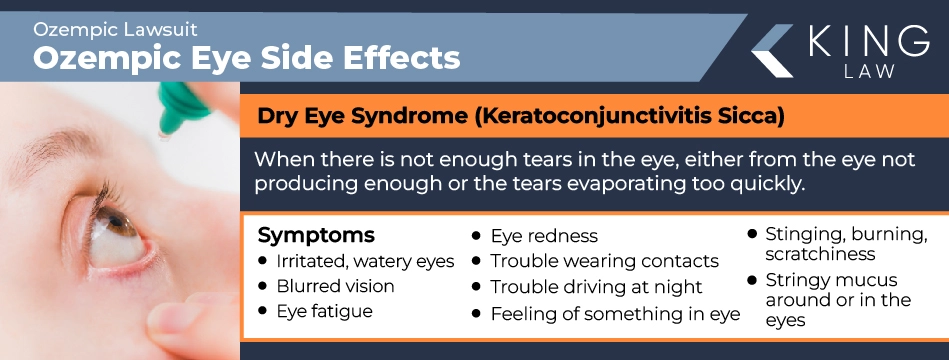
Dry Eyes
Dry eye syndrome (keratoconjunctivitis sicca) is when the eyes either don’t produce enough tears or tears evaporate too quickly, leading to dryness, inflammation, and potential damage to the eye’s surface. Symptoms of dry eyes can include:
- Eye stinging, burning, or scratchiness
- Stringy mucus around or in the eyes
- Light sensitivity
- Eye redness
- The sensation of foreign bodies in the eyes
- Trouble wearing contacts
- Trouble driving at night
- Irritated, watery eyes
- Blurred vision
- Eye fatigue
Potential causes of reduced tear production, quickly evaporating tears, and tear composition imbalances include:
- Age
- Medical conditions like diabetes, rheumatoid arthritis, and thyroid disorder
- Medications like antihistamines, decongestants, and blood pressure medications
- Wind, smoke, or dry air
- Infrequently blinking, such as when reading or staring at a screen
- Eyelid problems
- Imbalances with the oil, water, and mucus tear layers
While dry eye is not a commonly listed side effect of Ozempic, it could be indirectly linked. Ozempic may cause dehydration, changing blood sugar levels, hormone-like effects that impact bodily fluids, and systemic inflammation.
- Ozempic can cause nausea, vomiting, and diarrhea which can lead to dehydration and fewer tears
- Ozempic-triggered fluctuations in blood sugar levels can impact tear production and corneal health
- Ozempic’s hormone-like effects can change bodily fluids, reducing tear production.
- Ozempic could trigger systemic inflammation that impacts the ocular surface.
Eye Twitching
Eye twitching or eyelid twitching (myokymia) is when the eyelid muscles spasm involuntarily and repeatedly. The twitching is usually temporary and in the upper lid, but it can impact the lower lid and be quite bothersome. Eye twitching may last for just a few seconds or a minute, and may recur for days or weeks. Potential causes of eye twitching include:
- Increased stress levels that cause muscle spasms
- General fatigue or tiredness from lack of sleep
- Consuming too much caffeine
- Eye strain from too much screen time or visually straining tasks
- Eye irritation and dryness
- Nutritional imbalances such as magnesium deficiency
- Allergies with irritation and inflammation
Ozempic may be connected to eye twitching by:
- Causing nausea, vomiting, and diarrhea that can lead to low electrolytes, magnesium deficiency, and dehydration
- Changing blood sugar levels that impact nerve and muscle function
- Correlation with the stress and fatigue associated with diabetes management and taking medication
- Contributing to drug interactions
Additional Eye Issues From Ozempic
Other potential eye issues that patients taking Ozempic may experience include:
- Allergic reactions: If someone is allergic to Ozempic, they could experience eye itching, watering, swelling, redness, and irritation.
- Blurry vision: Changing blood glucose levels from taking Ozempic can change the eye’s lens shape.
- Conjunctivitis (pink eye): Medications can change immune health and increase the risk of infections, such as with the conjunctiva.
- Diabetic retinopathy: High blood sugar levels that require medications like Ozempic can damage blood vessels in the retina.
- Macular edema: Poorly controlled diabetes can cause fluid to leak into the macula, causing swelling in the area responsible for vision detail.
- Ocular migraines: Medication can cause stress, hormonal changes, vision changes, and other circumstances that cause migraines.
Visual disturbances: Changes to blood sugar when taking Ozempic can cause floaters, light sensitivity, and color perception shifts.
New Study Links Ozempic and Rare Eye Disease NAION
The first study connecting Ozempic to blindness looked at the medical records of more than 16,000 patients from 2017 to 2023 after Ozempic was released. Some patients were taking Ozempic for diabetes and others for weight management. Both groups were compared to identify a potential correlation between semaglutide and NAION.
Researchers concluded, “Our main finding is that prescribed semaglutide is associated with an increased risk of NAION.” Diabetic patients who took semaglutide were more than four times more likely to develop NAION than those on non-GLP-1 medications. Obese patients who took semaglutide were more than eight times more likely to develop NAION than those on non-GLP-1 medications.
While the study didn’t confirm that Ozempic directly causes NAION, it was significant enough for researchers to suggest patients be warned about the potential side effect. Researchers also acknowledged the need for more research that could include a larger, randomized sample group for a longer period.
Percentage of Ozempic Users Reporting Blindness
Study results indicate an increased risk of non-arteritic anterior ischemic optic neuropathy (NAION) in patients using semaglutide for Type 2 diabetes and obesity. These conclusions are based on a study involving 710 patients with Type 2 diabetes, highlighting the substantial risk associated with semaglutide.
- Taking semaglutide to treat type 2 diabetes is associated with a four times higher risk of developing NAION compared to other medications.
- Specifically, 8.9% of diabetic patients who were administered semaglutide developed NAION, in contrast to only 1.8% of patients taking other medications.
- Taking semaglutide for obesity treatment has an incidence rate of NAION seven times higher than with other medications.
- Specifically, in the group of patients prescribed semaglutide for weight loss, 6.7% developed NAION compared to just 0.8% of those on other treatments.
These findings showcase the need for careful consideration and monitoring of patients prescribed semaglutide, given the elevated risks of serious eye conditions associated with its use.
Ozempic Manufacturer’s Response to NAION Allegations
Novo Nordisk, like many other pharmaceutical companies that have faced backlash for not disclosing serious health risks, has pushed back against such claims. News outlets have reported that the company’s response has been “unperturbed,” dismissing concerns about potential blindness linked to Ozempic and reiterating that the data is insufficient to show a strong connection between semaglutide and NAION.
A Novo Nordisk spokesperson pointed out that NAION is not listed on approved labels as an “adverse drug reaction for the marketed formulations of semaglutide.” They also noted limitations in the Mass Eye and Ear study, such as its small and non-randomized sample group. The spokesperson emphasized that “patient safety is a top priority for Novo Nordisk, and we take all reports about adverse events from our medicines very seriously.”
How to File an Ozempic Blindness Lawsuit
To file an Ozempic blindness personal injury lawsuit:
- Set up an initial consultation with an experienced attorney to review your case and confirm eligibility. They will also help you understand the timeline for when you must file to be eligible for compensation.
- Begin gathering evidence to prove you took Ozempic and developed NAION.
- Be available to your attorney as they build and file your case with the appropriate court.
- Follow your attorney’s guidance if presented with an option to reach a settlement, negotiate, or go to trial.
- If a settlement isn’t reached, your case will be presented in trial.
- If the case settles in your favor, you will receive compensation that can be used for medical bills and other damages.
Again, be prepared to collect as much evidence as possible such as medical records, testimonies, doctor’s notes, medical bills, prescription information, and more to detail your experience taking Ozempic and resulting health issues plus their financial impact.
Contact an Ozempic Lawyer for Vision Loss
Reach out to an Ozempic lawyer like King Law as soon as possible. Filing an Ozempic lawsuit requires adherence to state-specific statutes of limitations, which often only allow 2-3 years from the date of your diagnosis or injury to file a claim. To ensure you don’t miss your opportunity for justice and compensation, it is crucial to act promptly.
Contact a lawyer who has extensive experience in handling cases involving Ozempic or other semaglutide drugs, particularly those linked to serious side effects such as blindness, stomach paralysis, and intestinal blockages. An experienced lawyer can guide you through the legal process, helping you navigate the complexities of your case and maximize your chances of a successful outcome.
Frequently Asked Questions (FAQs)
Learn more about how Ozempic impacts the eyes with answers to these frequently asked questions.