
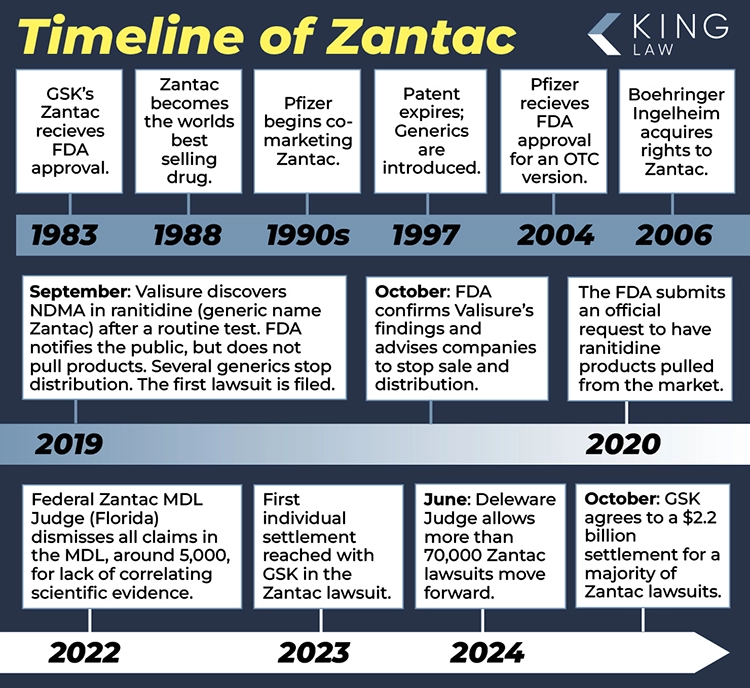
Thousands of people nationwide have filed lawsuits against the manufacturers of Zantac, claiming the popular acid-reducing drug caused them to develop cancer. Zantac, whose active ingredient is ranitidine, is no longer on the market. The FDA requested the removal of Zantac from the market in April of 2020. The FDA made this request after an investigation showed medications containing ranitidine may also contain a contaminant known as N-Nitrosodimethylamine (NDMA). NDMA is a probable human carcinogen known to cause certain kinds of cancer, including bladder, stomach, and esophageal cancers. People who have developed cancer after taking Zantac or other medications containing ranitidine are encouraged to contact an attorney. Litigation against the manufacturers of Zantac continues, with cases in various stages of the legal process. People who took Zantac may develop cancer later in life, so speaking with an attorney as soon as possible is crucial to determine if they qualify for a lawsuit.
Zantac Lawsuit Overview
Zantac (ranitidine) was a popular medication that treated heartburn and acid reflux by reducing stomach acid production. It was commonly taken in tablet form and was available over the counter and by prescription. The drug was widely used for decades before being recalled due to N-nitrosodimethylamine (NDMA) contamination concerns.
Prolonged use of ranitidine-containing drugs has been associated with an increased risk of developing cancers such as bladder, stomach, esophageal, liver, and colorectal cancer. Many of these are potentially life-threatening conditions, requiring expensive treatments and resulting in long-term physical and emotional suffering.
Lawsuits nationwide allege that the manufacturers of Zantac, including GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Sanofi, and Patheon, failed to warn patients and healthcare professionals about the risks of developing cancer. The lawsuits allege manufacturers knew the drug could be contaminated with NDMA. NDMA is a probable human carcinogen that may form in ranitidine. Despite being aware of the potential dangers associated with the medication, consumers say manufacturers prioritized profits over consumer safety. Most Zantac lawsuits focus on two manufacturers: Sanofi and Boehringer Ingelheim.
The lawsuits state the original product labels on the medication did not mention the risk of NDMA contamination or cancer. Additionally, they allege that manufacturers took far too long to issue recalls after discovering NDMA contamination and that adverse event reports were overlooked or downplayed. Due to widespread Zantac use and the number of affected individuals, many cases have been consolidated in federal court under multidistrict litigation (MDL). MDL 2924, In Re: Zantac (Ranitidine) Products Liability Litigation, was established in February of 2020 and is still accepting claimants.
Victims who developed cancer after using Zantac for prolonged periods may be eligible for compensation and are encouraged to contact King Law to schedule a free consultation. Our experienced attorneys can assist with filing claims and obtaining compensation for medical expenses, lost wages, and other damages.
Zantac Lawsuit – 2025 Update
March 25, 2025: Distinguished Legal Groups File Brief in Support of Plaintiffs in Delaware Zantac Appeal
Pharmaceutical companies GSK, Boehringer Ingelheim, and others have filed an appeal with the Delaware Supreme Court. The appeal challenges a lower court’s decision in favor of the plaintiffs, and it has been pending for several months now. In hopes of defeating this appeal, several legal organizations, including the Delaware Trial Lawyers Association, have filed a brief in support of the consumers who got cancer and other diseases because of the NDMA in Zantac.
March 4, 2025: Zantac Cases Increase; Jury Rules in Favor of Drug Manufacturer in Two Illinois Retrials
A Chicago jury found in favor of drug manufacturer Boehringer Ingelheim in the retrial of two Zantac lawsuits. The plaintiffs may appeal this verdict. These two lawsuits are just part of the larger litigation involving NDMA in Zantac heartburn products. There are 2,965 cases pending in the active multidistrict litigation against drug companies that made the brand and generic versions of Zantac. These drugs were recalled because their active ingredient degrades into the cancer-causing NDMA when stored in humid or hot environments. The ruling issued by the Chicago juries underscores the importance of retaining a reputable attorney who understands these cases and will appeal outcomes when necessary.
February 5, 2025: New Zantac Trial Occurs in Illinois Court as Delaware Appeal Continues
Two Illinois men who allege Zantac caused them to develop cancer pressed their cases on in a new trial in state court. At trial, the court heard evidence of repeated instances of the drug’s manufacturer—Boehringer Ingelheim—ignoring “red flags” of the risk of cancer due to its ingredients. Meanwhile, Boehringer Ingelheim and other drug manufacturers—including Pfizer—filed an appeal in Delaware to challenge rulings by judges in other Zantac cases. People who developed cancer after taking Zantac for heartburn are still filing lawsuits against manufacturers.
December 9, 2024: Evidence Suggests NDMA Levels in Zantac Increased Due to High Temperatures and Humidity
After Zantac was recalled from the market, many researchers tried to understand how a drug could leave a factory with acceptable levels of NDMA but later contain unsafe levels of the chemical. A research article published in the Journal Organic Process Research & Development found that common storage conditions increased the amount of NDMA found in Zantac. When ranitidine-containing drugs are exposed to humidity and high temperatures, the level of NDMA usually increases as certain molecules in the drug degrade. So, when people stored Zantac in their bathrooms or vehicles, they could have unknowingly increased NDMA levels, putting themselves at risk of cancer and other conditions.
December 3, 2024: New Peer-Reviewed Study Examines How Zantac Becomes Contaminated With NDMA
A December 2024 study published in the Journal of Pharmaceutical and Biomedical Analysis Open examined how Zantac (ranitidine) products become contaminated with NDMA (N-nitrosodimethylamine). Researchers in Japan examined two possible ways NDMA, which is a mutagenic carcinogen, was present in a pill that was intended to prevent heartburn. Their research suggested two ways the impurity occurred: NDMA formation by decomposition during storage and NDMA formation during the manufacturing process. Understanding how this impurity occurred could be important in protecting patients from similar contaminations. People who took ranitidine drugs contaminated with NDMA have filed more than 2,000 lawsuits against drug manufacturers alleging the drugs caused cancer and other diseases.
November 27, 2024: Trial for Man Who Says Zantac Caused His Bladder Cancer Ends Without a Verdict
A man who filed a lawsuit against Zantac manufacturer Boehringer Ingelheim had his trial end with a hung jury. On November 21, 2024, the jury was split 6 to 6 on whether or not Zantac was responsible for causing John Russell’s bladder cancer. However, the jury did find that Boehringer Ingelheim failed to adequately warn Zantac consumers about the dangers of the drug. The case will have to be retried, but it is promising that the jury found Boehringer Ingelheim to be negligent in warning people that Zantac could be dangerous, as the degrades into probable carcinogenic compounds within the human body.
November 21, 2024: Zantac Settlements From GSK Could Paid Out by June 2025
Plaintiffs who agree to the terms of the settlement agreement with GlaxoSmithKline (GSK) may receive their compensation by June 2025. In October, GSK reached a $2.2 billion settlement agreement with law firms who represented 80,000 plaintiffs who were harmed by contaminated ranitidine. For plaintiffs who were offered settlements, they have the choice to accept those settlements. If they accept the terms of the settlement, they may receive payment mid next year. In GSK’s press release about the settlement, they said, “The participating plaintiff firms are unanimously recommending to their clients that they accept the terms of the State Courts Settlement, which is expected to be fully implemented by the end of H1 2025.” As with most settlements, the amounts are confidential.
November 15, 2024: Woman Files Lawsuit Alleging 11 Years of Zantac Use Caused Her Breast Cancer
A Californian woman filed a lawsuit against Zantac manufacturer GlaxoSmithKline alleging that years of Zantac (ranitidine) use led to her breast cancer diagnosis. Cynthia Vanantwerp took brand-name Zantac and generic equivalents from 1997 to 2018. Her attorneys allege that ingestion of ranitidine-containing products caused their client to be exposed to N-Nitrosodimethylamine (NDMA), which is a potent carcinogen. She says this exposure led to her breast cancer diagnosis and that GlaxoSmithKline knew or should have known about the connection between NDMA exposure and developing cancer. Vanantwerp’s case has been transferred to the Southern District of Florida as part of a multidistrict litigation against Zantac manufacturers (MDL 2924).
November 7, 2024: Zantac Lawyers Continue To Take Cases From People Harmed by Ranitidine
The number of pending cases in the Zantac multidistrict litigation (MDL 2924) grew from October to November, which indicates attorneys are still filing Zantac lawsuits on behalf of their clients. As time progresses, more people are realizing their digestive cancers may be linked to their use of Zantac. People who developed cancers of the stomach, bladder, esophagus, colon, rectum, liver, pancreas, or kidneys after taking Zantac may be eligible to file a case. We are still filing lawsuits for our clients.
November 1, 2024: More Plaintiffs Join Federal Zantac Lawsuit in October
As of November 1, 2024, there were 2,427 pending lawsuits against ranitidine manufacturers, including Sanofi, GlaxoSmithKline, Pfizer, Boehringer Ingelheim, and Chattem Inc. Many people who have experienced cancer and other serious side effects after taking ranitidine-containing products have consolidated their cases in a multidistrict litigation (MDL-2924, Zantac (Ranitidine) Products Liability Litigation). People are still filing lawsuits against these manufacturers, as those people discover their cancers may have been caused by Zantac that contained a probable carcinogen called NDMA.
October 2024: GlaxoSmithKline (GSK) agreed to pay up to $2.2 billion to settle the majority of lawsuits related to Zantac. The settlement would help to resolve approximately 80,000 state court cases.
September 2024: Jury selection is expected to begin in California’s first Zantac trial out of the Alameda County Superior Court in Oakland. While many manufacturers have settled Zantac lawsuits, this will help to set a precedent for future litigation.
August 2024: Tens of thousands of cases have been filed across the country alleging that the manufacturers of Zantac knew or should have known the increased risk of cancer associated with use of the drug.
About the Zantac Lawsuit:
Zantac Side Effects and Health Risks
Zantac and Cancer: High NDMA Levels Found in Zantac
Types of Cancers Caused by Zantac
Zantac Manufacturers Named in the Lawsuit
Eligibility Criteria in the Zantac Lawsuit
Evidence Needed to File a Zantac Lawsuit
Recoverable Damages in the Zantac Lawsuit
Zantac Lawsuit Settlement and Payout Amounts
What Is Zantac?
Zantac is a brand name for ranitidine, a histamine-2 (H2) blocker used to treat conditions involving excess stomach acid. The drug did this by blocking histamine from stimulating acid production in the stomach lining and therefore reducing the amount of acid produced by the stomach.
It was also used to treat and prevent ulcers in the stomach and intestines, manage excess acid production conditions like Zollinger-Ellison syndrome, and address reflux conditions such as gastroesophageal reflux disease (GERD), heartburn, and acid reflux.

How Does Zantac Work?
Zantac blocks histamine-2 (H2) receptors in the stomach lining to reduce acid production. It was used to treat conditions like heartburn, GERD, peptic ulcers, and Zollinger-Ellison syndrome by reducing acid irritation and promoting healing. The medication worked by preventing histamine from triggering stomach acid secretion and lowering acidity in the stomach and esophagus. Effects start within 30 minutes and last up to 12 hours.
Conditions Zantac Treats:
- Heartburn (Acid Indigestion): Relieves symptoms by reducing esophageal irritation.
- Gastroesophageal Reflux Disease (GERD): Prevents acid from backing up into the esophagus.
- Peptic Ulcers: Promotes healing by reducing stomach and intestinal acid irritation.
- Zollinger-Ellison Syndrome: Manages excessive acid production, leading to severe ulcers.
Ultimately, Zantac prevented the worsening of acid-related issues, decreased acid production to avoid further damage, facilitated healing, and allowed ulcers and irritated tissues time to recover.
Zantac Side Effects and Health Risks
Common side effects of Zantac include mild gastrointestinal issues, neurological symptoms, and skin reactions. However, use of the medication is also associated with serious side effects that may require medical attention. The side affects included allergic reactions, cardiovascular effects, liver dysfunction, blood disorders, respiratory issues, mental changes, vision changes, and other severe health conditions.
Common Side Effects of Zantac:
- Gastrointestinal Issues
- Nausea or vomiting
- Diarrhea or constipation
- Stomach pain or cramps
- Loss of appetite
- Neurological Symptoms
- Headache
- Dizziness
- Drowsiness
- Fatigue
- Insomnia
- Rash or skin irritation
These common side effects are generally mild and often resolve on their own, but they should be reported to your healthcare provider for monitoring.
Serious Side Effects of Zantac:
- Allergic Reactions: Symptoms include rash, itching, and swelling of the face, lips, tongue, or throat. An allergic reaction may also cause severe dizziness and difficulty breathing.
- Cardiovascular Effects: Symptoms may include Irregular heartbeat (fast or slow heartbeat) and chest pain.
- Liver Dysfunction: Signs of serious liver problems include yellowing of the skin or eyes (jaundice), dark urine, pale or clay-colored stools, and unusual tiredness.
- Blood Disorders: Symptoms may include easy bruising or bleeding and fever.
- Respiratory Issues: May present as shortness of breath, cough with mucus, and an increased risk of pneumonia.
- Mental Changes (Especially in older adults): Symptoms include confusion, agitation, hallucinations, and vision Changes.
- Other Reported Side Effects: Such as appetite loss, fatigue, and problems with skin or hair.
Many of these serious side effects of Zantac have long-term health implications and may require medical attention. Any new or worsening conditions should be immediately reported to your healthcare provider.
Zantac and Cancer: High NDMA Levels Found in Zantac
N-nitrosodimethylamine (NDMA) is a chemical classified by the Environmental Protection Agency (EPA) as a probable human carcinogen. Health risks associated with NDMA include an increased risk of several types of gastrointestinal cancers. Several studies showed a link to various cancers in laboratory animals. A study of data from the FDA’s Adverse Event Reporting System (FAERs) looked at outcomes in human patients who were taking ranitidine. That study, called, “The Association between Ranitidine Use and Gastrointestinal Cancers,” concluded that NDMA does indeed raise the risk of gastric cancers in humans. It stated, “The results of the current study provided direct support for the assertion that NDMA contaminated ranitidine is associated with the occurrence of gastrointestinal cancer.”
Testing of ranitidine-containing drugs found that levels of NDMA can increase over time and when ranitidine is stored at higher temperatures. The instability of the ranitidine molecule can lead to NDMA formation under certain conditions, such as heat and digestion. However, even under normal storage conditions, NDMA levels in ranitidine can rise over time.
H3: The FDA’s Response to NDMA in Ranitidine Products
In September 2019, the U.S. Food and Drug Administration (FDA) issued a warning about NDMA in ranitidine products. Tests by the FDA and independent laboratories detected NDMA contamination. In response to the findings, major manufacturers like Sanofi, GlaxoSmithKline, and Sandoz, Inc. initiated voluntary recalls of ranitidine-containing products.
In April 2020, the FDA requested the withdrawal of all ranitidine products from the U.S. market. The FDA advised consumers to stop taking over-the-counter ranitidine and dispose of any remaining products.
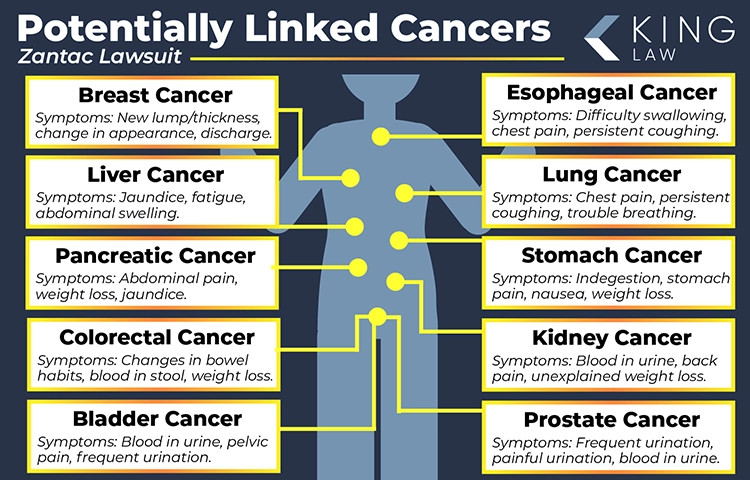
Types of Cancers Caused by Zantac
Zantac has been linked to several types of cancers due to NDMA contamination, including stomach cancer, bladder cancer, esophageal cancer, colorectal cancer, liver cancer, pancreatic cancer, kidney cancer, and other potentially linked cancers such as prostate, breast, and lung cancer.
Cancers Potentially Caused by Zantac and Their Symptoms:
Stomach Cancer (Gastric Cancer) Symptoms
- Indigestion
- Stomach pain
- Nausea
- Unexplained weight loss
Bladder Cancer
- Blood in the urine
- Frequent urination
- Pelvic pain
Esophageal Cancer
- Difficulty swallowing
- Chest pain
- Persistent coughing
Colorectal Cancer (Colon and Rectal Cancer)
- Changes in bowel habits
- Blood in the stool
- Unexplained weight loss
Liver Cancer
- Jaundice (yellowing of the skin or eyes)
- Fatigue
- Abdominal swelling
Pancreatic Cancer
- Abdominal pain
- Weight loss
- Jaundice
Kidney Cancer
- Blood in the urine
- Back pain
- Unexplained weight loss
Other Potentially Linked Cancers
- Prostate Cancer
- Breast Cancer
- Lung Cancer
Any new or worsening symptoms should be reported to your healthcare provider to ensure prompt diagnosis and treatment.
Zantac Manufacturers Named in the Lawsuit
Several manufacturers, including Sanofi, GlaxoSmithKline (GSK), Pfizer, Boehringer Ingelheim, and Chattem Inc., have been named in the Zantac lawsuit due to allegations of failure to warn about NDMA contamination risks, negligence in testing and distributing ranitidine, and design defects leading to NDMA formation.
Zantac Manufacturers:
- Sanofi: Major manufacturer and marketer of over-the-counter Zantac in the U.S.
- GlaxoSmithKline (GSK): Originally developed and marketed Zantac in the 1980s.
- Pfizer: Co-marketed prescription Zantac in the U.S. during the late 1990s and early 2000s.
- Boehringer Ingelheim: Acquired U.S. rights to Zantac from GSK in 2006 and marketed the drug for several years.
- Chattem Inc.: Involved in the marketing and distribution of Zantac products.
Allegations against manufacturers include that the companies failed to warn consumers and healthcare providers about the dangers associated with the use of the drug, that they knew or should have known about NDMA contamination risks, they were negligent in the manufacturing, distributing, and marketing of the drug, and they engaged in inadequate testing to identify NDMA risks in ranitidine.
Other accusations against the manufacturers include that there was a design defect in the drug and that it is inherently unstable, potentially breaking down into NDMA under certain conditions.
Generic Manufacturers:
- Teva Pharmaceuticals
- Amneal Pharmaceuticals
- Apotex Corp.
- Perrigo
- Dr. Reddy’s Laboratories
- Sandoz (Novartis’ generic division)
In July 2021, Judge Robin L. Rosenberg dismissed cases against generic drug makers because federal law prevents them from being sued over claims related to generic medications.
Eligibility Criteria in the Zantac Lawsuit
Eligibility criteria for a Zantac lawsuit require that individuals have used the brand-name Zantac for at least one year between 1983 and 2019. Additionally, eligible claimants must have been diagnosed with one of the following cancers at least one year after taking Zantac: bladder, liver, gastric/stomach, esophageal, pancreatic, lung, colorectal, prostate, or breast cancer.
To be eligible for a Zantac lawsuit, claimants must:
- Have a cancer diagnosis of Bladder Cancer, Liver Cancer, Gastric/Stomach Cancer, Esophageal Cancer, Pancreatic Cancer, Lung Cancer, Colorectal Cancer, Prostate Cancer, or Breast Cancer.
- Have taken brand-name Zantac regularly for a minimum of one year within the specified period (1983–2019).
- The cancer diagnosis must have occurred at least one year after initial Zantac use.
Claimants must have used brand-name Zantac within the United States and be U.S. citizens. Evidence of Zantac usage prior to the cancer diagnosis is essential to establish causation. Some claims may have time limitations, so consulting an attorney promptly is advised.

Evidence Needed to File a Zantac Lawsuit
Substantial evidence is needed to file a Zantac lawsuit, including medical records confirming cancer diagnosis and treatment history, proof of Zantac or ranitidine use through prescription records, receipts, or medication bottles, and a timeline of usage with dates and dosages If someone chooses to hire an attorney to file a Zantac lawsuit, they will work to establish things like: evidence of NDMA contamination such as lot numbers or FDA warnings, expert testimony from medical professionals and toxicologists, personal testimony detailing usage and lifestyle factors, research studies linking Zantac to cancer. Attorneys will also work with their clients to obtain documentation of financial losses like medical bills and lost wages, legal documents like insurance claims, and witness testimonies from family or caregivers about the impact of the illness.
Key Pieces of Evidence to Gather:
Medical Records
- Diagnosis of Cancer: Medical records confirming the type and stage of cancer (biopsies, scans, pathology reports)
- Treatment History: Documentation of treatments received (chemotherapy, radiation, surgery, follow-up care)
- Medical History: Complete medical history indicating no prior history of cancer before using Zantac
Proof of Zantac or Ranitidine Use
- Prescription Records: Pharmacy records showing the length of time and dosage of prescribed Zantac
- Receipts for Over-the-Counter Purchases: Receipts from pharmacies or stores, loyalty card data, online purchase history
- Medication Bottles: Physical evidence of Zantac or generic ranitidine packaging
Timeline of Use
- Dates of Zantac Usage: Detailed timeline of when and how long Zantac was taken
- Dosage Information: Evidence of the dosage strength regularly taken (e.g., 75 mg, 150 mg)
Evidence of NDMA Contamination
- Lot Numbers or Product Batches: Documentation of consumed lot numbers or batches linked to recalls
- FDA Warnings or Recalls: Records of FDA warnings and recalls related to NDMA contamination in Zantac
Expert Testimony
- Medical Expert: Testimony linking Zantac use to the cancer diagnosis, explaining NDMA’s effects
- Toxicologist: Expert insights on NDMA presence in Zantac and its carcinogenic impact
- Treating Physician Testimony: Statements from the patient’s own doctor regarding the case
Personal Testimony
- Statement of Zantac Use: Personal account detailing duration, frequency of use, and any symptoms experienced
- Lifestyle and Habits: Information showing lack of other risk factors (non-smoker, moderate alcohol consumption, no family history of cancer)
Research and Scientific Studies
- Studies on Zantac and Cancer: Scientific research linking NDMA contamination in Zantac to cancer
- FDA Reports: Official reports or studies confirming NDMA issues in Zantac products
Documentation of Financial Losses
- Medical Bills: Invoices for treatments, hospital stays, medications, and rehabilitation
- Lost Wages or Earnings: Evidence of income lost due to illness (pay stubs, employer statements)
- Out-of-Pocket Expenses: Receipts for travel, accommodations, or other treatment-related costs
Legal Documents
- Insurance Claims: Copies of claims related to the cancer diagnosis and treatment
Witness Testimony
- Family or Caregiver Statements: Accounts of the cancer’s impact on daily life and well-being
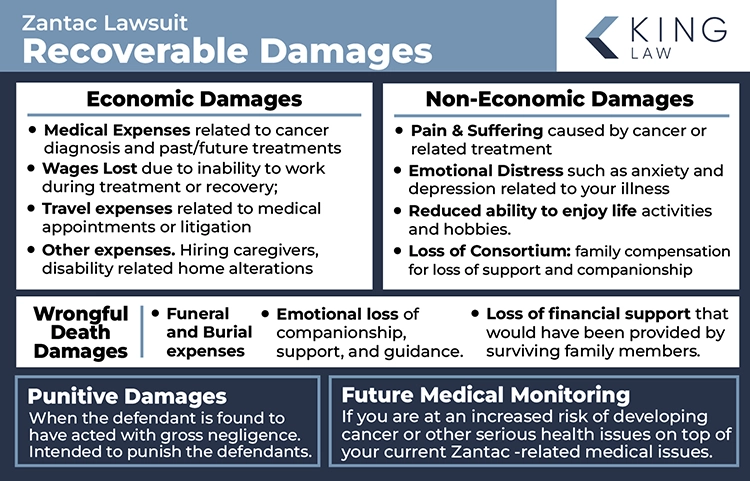
Recoverable Damages in the Zantac Lawsuit
Recoverable damages in the Zantac lawsuit include compensatory damages, economic and non-economic damages, punitive damages, wrongful death damages, and future medical monitoring costs. Factors affecting the amount of compensation someone receives may include the severity of the illness, strength of evidence linking cancer to Zantac use, and the economic impact of the illness. The plaintiff’s age, health, and life expectancy will also be considered.
Damages You Can Recover:
Economic Damages
- Medical Expenses: Costs for past and future treatments related to cancer diagnosis (hospital stays, surgeries, medications, chemotherapy, radiation, ongoing care).
- Lost Wages: Compensation for income lost due to inability to work during treatment or recovery; may include future lost earning capacity if disabled.
- Other Financial Losses: Expenses for traveling to medical appointments, hiring caregivers, or home modifications due to disability.
Non-Economic Damages
- Pain and Suffering: Compensation for physical pain and emotional suffering caused by cancer and its treatment.
- Emotional Distress: Psychological impacts like anxiety, depression, or mental anguish related to the illness.
- Loss of Enjoyment of Life: Reduced ability to enjoy life activities and hobbies previously enjoyed.
- Loss of Consortium: Compensation for spouses or family members for loss of companionship, support, or intimacy due to the plaintiff’s illness.
Punitive Damages: Awarded when manufacturers acted with gross negligence or willful misconduct. Punitive damages are intended to punish defendants and deter similar future conduct. They are typically awarded if manufacturers knowingly withheld information about NDMA contamination risks or acted with reckless disregard for consumer safety.
Wrongful Death Damages (Applicable if a plaintiff dies from cancer allegedly linked to Zantac)
- Funeral and Burial Expenses: Costs associated with funeral and burial services.
- Loss of Financial Support: Compensation for income and benefits the deceased would have provided to surviving family members.
- Loss of Companionship: Emotional loss of the deceased’s companionship, guidance, and support.
Future Medical Monitoring: Damages for future medical surveillance if at increased risk of developing cancer or other serious health conditions due to prolonged Zantac use.
Factors that may affect the amount of damages you receive include the severity of the illness (type and stage of cancer), strength of evidence linking cancer to Zantac use, plaintiff’s age, health, and life expectancy, and the economic impact of the illness.
How to File a Zantac Lawsuit
To file a Zantac lawsuit you will need to follow several steps starting with confirming eligibility and consulting with an experienced attorney. Throughout the process, it is important to work closely with your attorney to ensure a successful outcome in your claim.
Steps to File a Zantac Lawsuit:
- Confirm Eligibility
- Cancer Diagnosis: Ensure you have a confirmed diagnosis of a cancer type linked to Zantac use, such as bladder, stomach, esophageal, or another related cancer.
- Zantac Usage Verification: Collect proof of prolonged Zantac usage, including prescription history, pharmacy receipts, or purchase records that show you took the medication over a significant period.
- Consult with an Experienced Lawyer:
- Find a Lawyer: Seek out attorneys who are well-versed in pharmaceutical litigation or product liability, particularly those with experience in Zantac or similar drug-related cases.
- Free Case Evaluation: Many law firms offer free consultations to assess your case’s strength and discuss potential legal strategies.
- Gather and Prepare Necessary Documentation:
- Medical Records: Collect all relevant medical records that confirm your cancer diagnosis, including pathology reports, imaging results (MRIs, CT scans), and treatment history.
- Proof of Zantac Usage: Gather tangible evidence of your Zantac usage, such as pharmacy receipts, prescription records, or product packaging labels.
- Witness Statements: Compile statements from healthcare providers, family members, or friends who can confirm your Zantac use and the onset of symptoms.
- File the Lawsuit:
- Official Filing: Your attorney will draft and file a legal complaint against the manufacturers of Zantac in the appropriate court.
- Statute of Limitations: Ensure the lawsuit is filed within the legal time limits, which vary by state but typically range from one to several years from the date of diagnosis or discovery of the cancer link.
- Discovery Phase:
- Exchange of Evidence: Both sides will exchange relevant information and evidence, including medical records, expert testimonies, and depositions.
- Expert Testimonies: Both parties may hire medical and pharmacological experts to testify about the link between Zantac and your cancer diagnosis.
- Settlement Negotiations:
- Negotiation Process: Before trial, there is often an attempt to settle the case out of court. Your lawyer will negotiate with the manufacturers’ representatives to secure fair compensation.
- Evaluate Settlement Offers: Review all settlement offers with your attorney to determine if they sufficiently cover medical expenses, lost income, pain and suffering, and other damages.
- Trial:
- Going to Court: If a settlement cannot be reached, your case may proceed to trial, where your attorney will present your evidence to a judge or jury.
- Presentation of Evidence: Both sides will present their arguments and evidence, and a verdict will be made based on the case’s merits.
- Post-Trial:
- Appeals Process: If the trial outcome is unfavorable, you may have the option to appeal the decision.
- Collection of Damages: If you win the case, steps will be taken to collect the awarded damages.
- Monitor Health and Follow-Up:
- Ongoing Medical Care: Continue to monitor your health and receive ongoing medical care as needed for your condition.
- Legal Follow-Ups: Maintain communication with your lawyer for any post-trial follow-ups or to manage the appeals process if necessary.
Zantac Lawsuit Settlement and Payout Amounts
Zantac lawsuit settlement and payout amounts vary depending on the type of injury and the manufacturer involved. Specifically, average payouts may vary by injury tier. Tier I injuries, including individuals diagnosed with stomach, prostate, pancreatic, or breast cancer, may potentially receive higher settlements than Tier II injuries which include individuals diagnosed with liver, bladder, or kidney cancer. King Law’s estimated range of settlement amounts for Zantac lawsuits is between $300,000 and $500,000.
Previous Zantac Settlements by Companies:
- GlaxoSmithKline (GSK): Agreed to pay up to $2.2 billion to settle most state court lawsuits in the U.S. claiming that Zantac causes cancer.
- Sanofi: This settlement paid $100 million to settle Zantac cancer lawsuits. Provided an average of more than $25,000 per claim.
- Pfizer: Offered up to $250 million to settle more than 10,000 U.S. lawsuits over Zantac’s cancer risks.
Anticipated Average Payouts by Injury Tier:
- Tier I Injuries: Includes cancers such as stomach, prostate, pancreatic, or breast cancer with likely payout range between $300,000 and $500,000.
- Tier II Injuries: Includes cancers such as liver, bladder, or kidney cancer with likely payout range between $100,000 and $250,000.
- Tier III Injuries: Other injuries have a likely payout range between $30,000 and $75,000.
Zantac Lawsuit Statute of Limitations and Deadlines
A statute of limitations is a law that sets the maximum time after an event within which legal proceedings may be initiated. The statute of limitations for filing a Zantac lawsuit varies by state, typically ranging from one to six years after a cancer diagnosis or when the injury was discovered.
States with a 1-Year Statute of Limitations:
- Kentucky
- Louisiana
- Tennessee
States with a 2-Year Statute of Limitations:
- Alabama
- Alaska
- Arizona
- California
- Colorado
- Delaware
- Georgia
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Nevada
- New Jersey
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Texas
- Utah
- Virginia
- West Virginia
States with a 3-Year Statute of Limitations:
- Arkansas
- Connecticut
- District of Columbia
- Maryland
- Massachusetts
- Michigan
- Mississippi
- Montana
- New Hampshire
- New Mexico
- New York
- North Carolina
- Rhode Island
- South Carolina
- South Dakota
- Vermont
- Washington
- Wisconsin
States with a 4-Year Statute of Limitations:
- Florida
- Minnesota
- Nebraska
- Wyoming
State with a 5-Year Statute of Limitations:
- Missouri
States with a 6-Year Statute of Limitations:
- Maine
- North Dakota
The statute of limitations generally starts from the date of injury, harm, or cancer diagnosis. It’s crucial to consult a lawyer promptly to determine the specific statute of limitations applicable to your case, as exceptions and individual circumstances may affect these time frames.
Contact a Zantac Lawyer
If you have been diagnosed with cancer after taking Zantac for at least six months you may be eligible for a lawsuit. The attorneys at King Law have extensive experience handling drug defect and pharmaceutical liability cases. Contact King Law today to schedule a free consultation. Our legal team will work strategically to secure the compensation our clients deserve. We advocate for those affected by Zantac, ensuring that each case is handled with expertise and care, from gathering evidence to navigating settlement negotiations.

