
Veozah, a non-hormonal medication prescribed to treat menopausal hot flashes, has been linked to rare cases of severe liver injury. Individuals nationwide are filing lawsuits against the manufacturer of Veozah, claiming the company knew or should have known about the potential for harm associated with the use of the drug. While litigation is in the early stages, additional lawsuits are expected to be filed in the coming weeks and months due to the widespread use of prescription medication.
Veozah Lawsuit Overview
In May 2023, the U.S. Food and Drug Administration (FDA) approved Veozah. Veozah (fezolinetant) is the first non-hormonal medication approved to treat menopausal hot flashes. It works by blocking neurokinin B, a brain chemical that triggers hot flashes.
In September 2024, the FDA issued a warning about the potential for the drug to cause rare, but severe liver damage when taking the medication. One case reported liver damage after only 40 days of use. The regulatory agency also added new recommendations for Veozah patients, including increasing the frequency of liver function testing while taking the drug and stopping the prescription if signs and symptoms of liver injury occur. Discontinuation of Veozah may prevent liver damage from worsening and allow liver function recovery.
Veozah patients who are diagnosed with liver injury after taking the medication may be entitled to compensation by filing a lawsuit against the manufacturer. Compensation may cover medical bills, pain and suffering, and more. Aggrieved individuals are encouraged to contact King Law to schedule a free case review.
Veozah Lawsuit – 2025 Update
April 2, 2025: UK Health Officials Publish Updated Warnings About Veozah and Liver Injury
More international government agencies are expressing concern on the possible injuries caused by the hot-flash drug Veozah (fezolinetant). In early 2025, the UK required Astellas (the maker of Veozah) to issue updated warnings about the risk of liver injury when taking Veozah. According to the notice, physicians should perform liver function tests to make sure patients’ serum numbers are not too high before beginning treatment. Astellas and the UK health officials also encourage doctors to routinely reassess patients’ levels and monitor for signs of liver damage or failure—such as jaundice or elevated blood serum levels. People continue to file lawsuits after suffering liver injuries from Veozah.
March 25, 2025: Canada Approves Hot Flash Drug Veozah as Women in United States Report Liver Injuries
The Canadian government recently approved a 45-milligram regimen of Veozah to treat some women who are experiencing hot flashes because of menopause. Canadian officials acknowledged the increased risk of liver injury for some taking this drug. Meanwhile, American women are continuing to file lawsuits after suffering severe side effects, such as liver injury, from this non-hormonal hot flash treatment.
March 4, 2025: Women Continue To File Lawsuits Against Veozah Manufacturers
Three months after the FDA updated the Black Box warning label on Veozah (fezolinetant), affected women continue to file lawsuits. Reports from patients and researchers suggest using Veozah increases a woman’s chance of serious liver injuries and a cancer diagnosis. When it was first released, Veozah was hailed as a revolutionary non-hormonal hot-flash drug. Fezolinetant has been prescribed to women to treat vasomotor symptoms (VMS) brought on by menopause and breast cancer treatments.
February 5, 2025: Consumers Encouraged To Report Serious Side Effects of Veohzah to Doctors and the FDA
After the FDA elevated its warnings about Veozah, consumers were encouraged to report side effects to their doctors and the FDA. Consumers can report and view adverse reactions to Veozah and its generic version (fezolinetant) on the FDA’s MedWatch system. The FDA’s Adverse Event Reporting System (FAERS) Public Dashboard shows over 700 adverse reactions were reported by people taking Veozah and fezolinetant in 2024, including negative changes in someone’s liver function. Many people who have experienced liver damage after taking Veozah have filed lawsuits against Astellas Pharma, the maker of the hot-flash drug.
December 18, 2024: FDA Updates Veozah’s Black Box Warning to Highlight the Risk of Severe Liver Injury
On December 16, 2024, the U.S. FDA added additional information about the risk of severe liver injury to Veozah’s black-box warning. The menopause drug, which is used to reduce hot-flashes, now carries an updated “Boxed Warning” for risks of hepatotoxicity (liver toxicity). The Boxed Warning is the FDA’s most prominent warning, which is located at the top of the drug’s prescription information. The updated warning also tells people taking Veozah (fezolinetant tablets) to have regular blood tests to monitor liver function and to watch for symptoms such as fatigue. This label update occurs as more women are filing lawsuits against Veozah’s maker, Astellas Pharma, Inc, after experiencing liver toxicity from taking the drug.
December 12, 2024: Studies Reveal that Veozah Is Associated With Increased Tumor and Liver Injury Risk
For many women experiencing menopause, Veozah provided much-needed relief from debilitating hot flushes. However, research shows that women taking Veozah were up to 4.3 times more likely to receive a cancer diagnosis, although the link is still being studied. Even though fezolinetant is still given to women, scientists encourage caution due to the elevated risk of liver injury when taking this VMS drug.
December 2, 2024: Despite Risks of Liver Injuries, Researchers Encourage Use of Veozah in Some Men with Prostate Cancer
A research article published in the journal Translation Andrology and Urology encouraged using Veozah (fezolinetant) to treat hot flashes in some men with prostate cancer. The researchers report that up to 80 percent of men receiving androgen deprivation therapy—a treatment that reduces male hormones—experience hot flashes. The researchers suggest that Veozah could help relieve these symptoms. However, many studies link Veozah to severe liver injury, meaning men could be at risk of liver injuries from this menopause drug.
November 22, 2024: Researchers Caution Against Increased Tumor Risk When Using Veozah, A New Hot Flash Medication
A recently published study urges medical professionals and researchers to do additional research on the increased tumor risks when using Veozah. The study, called “Fezolinetant: a novel nonhormonal therapy for vasomotor symptoms due to menopause requiring a careful evaluation of the benefit-risk balance,” urges that more research is needed into the safety of the drug. Veozah is a first-of-its-kind non-hormonal hot flash drug that can help alleviate symptoms of hot flashes for women going through menopause. Despite pharmaceutical companies’ claims of Veozah’s safety, researchers warn that studies showed an increased risk of developing neoplasms— a type of tumor—when taking this drug at specific dosages.
November 15, 2024: Non-hormonal Hot Flash Drug, Veozah, Praised But Drug Increases Liver Damage Risks
Veozah, whose generic active ingredient is Fezolinetant, decreases hot flashes by addressing the imbalance of estrogen and Neurokinin B (NKB). Even so, government agencies caution against the increased risk of liver damage when taking Veozah. Researchers push Veozah as a “significant breakthrough” nonhormonal treatment for vasomotor (VSM) symptoms—hot flashes. Menopausal women may experience one hot flash per week or up to one to two per hour, which can impact a woman’s quality of life.
November 7, 2024: Researchers Warned of Veozah Causing Liver Damage, Call for Additional Safety Studies
According to LiverTox—part of the National Library of Medicine—clinical trials of fezolinetant—the active ingredient in Veozah—revealed that 2.3 percent of participants taking it had elevated liver enzyme levels. In that group of patients, their liver levels soared to three times the upper limit of normal. In other studies, researchers noted the need for a more critical review of the risks to women taking this “miracle” drug.
November 1, 2024: Veozah Continues to be Marketed as Safe and Effective Despite Possible Liver Damage
Veozah’s manufacturer, Astellas, markets the drug as a groundbreaking treatment for women experiencing hot flashes. Previously, women had fewer options to manage these symptoms. Some research studies said fezolinetant was “well-tolerated” by participants, but those studies have noted the possibility of liver damage for people taking fezolinetant, the active ingredient in Veozah. Many consumers developed severe liver damage after just over a month. Women who took Veozah for hot flashes and experienced a severe injury like liver damage may qualify to file a lawsuit.
October 16, 2024: FDA Recommends More Frequent Liver Monitoring for Women Taking Veozah
The U.S. Food and Drug Administration (FDA) is now recommending that women using Veozah (fezolinetant) have more frequent blood tests to monitor their liver function. The FDA required Veozah’s, manufacturer, Astella, to update the drug’s prescribing information. The prescribing information now states women should have their blood tested once a month for the first two months of Veozah use. The previous pamphlet only suggested physicians test liver-enzyme levels at 3, 6, and 9 of treatment. In September, the FDA also required Astella to add a warning about severe liver damage to Veozah’s label. As a result of possible liver damage, more women are expected to file lawsuits when experiencing liver problems after taking Veozah.
October 2024: Individuals who have been diagnosed with liver injury after taking Veozah are encouraged to contact an experienced lawyer to determine if they are eligible for compensation by taking legal action.
September 2024: The FDA issued a warning about the safety of Veozah. The warning noted that individuals taking the medication may be at an increased risk of developing a rare, but serious liver injury.
May 2023: Veozah receives FDA approval as the first non-hormonal medication approved to treat menopausal hot flashes.
About the Veozah Lawsuit:
FDA Adds ‘Serious Liver Injury’ to Veozah Warning Label
Veozah and Liver Damage Due to Elevated Liver Enzymes
Veozah Development, Approval, and Marketing Strategy
Eligibility Criteria in the Veozah Lawsuit
Evidence Needed to File a Veozah Lawsuit
Recoverable Damages in the Veozah Lawsuit
Veozah Lawsuit Statute of Limitations
FDA Adds ‘Serious Liver Injury’ to Veozah Warning Label
The FDA has made multiple updates to Veozah’s prescribing information and black-box warning label about the risks of severe liver disease. These updates warn people taking the menopause drug Veozah about the potential for drug-induced toxicity and liver damage. On December 16, 2024, the FDA updated Veozah’s black-box warning. The updated label warns people taking Veozah of the risks of hepatic toxicity, elevated liver enzymes, and warning signs of toxicity.
On September 12, 2024, the U.S. Food and Drug Administration issued a drug safety communication warning consumers about the potential of Veozah to cause a serious but rare type of liver injury. The warning indicated that the non-hormonal prescription drug used to treat menopausal hot flashes could cause liver damage. Symptoms of liver injury resulting from Veozah use include fatigue, nausea, jaundice, and dark urine.
The FDA also recommended that Veozah patients engage in increased liver function testing. The regulatory agency noted that liver testing should occur monthly for the two months after starting the medication and then at 3, 6, and 9 months of treatment, which was previously recommended. Furthermore, patients should stop taking the prescription drug if signs of liver injury occur to prevent worsening or further damage.
The FDA’s finding required that liver injury risk be added to Veozah’s prescribing information. Veozah patients should monitor for symptoms of injury damage and discontinue use of the drug if signs occur. Regular liver function tests should be conducted before and during treatment to ensure the patient’s health, and healthcare providers should inform patients about liver risks, testing requirements, and the need to stop the drug if signs of liver damage are present.
What Is Veozah?
Veozah is a non-hormonal prescription medication developed by Astellas Pharma, Inc. to treat moderate to severe vasomotor symptoms (VMS) like hot flashes and night sweats during menopause. It targets and blocks the neurokinin 3 receptor to regulate body temperature and restores the balance between estrogen and neurokinin B (NKB), reducing hot flashes during menopause. It is available as a brand-name oral tablet that was approved by the FDA in 2023.
The once-daily tablet was developed by Japanese pharmaceutical company Astellas Pharma. It offers an alternative to hormone replacement therapy (HRT) for women who are unable or unwilling to use hormones. The active ingredient in Veozah is fezolinetant. There is currently no generic available on the market. While it requires a prescription, it is not considered a controlled substance.
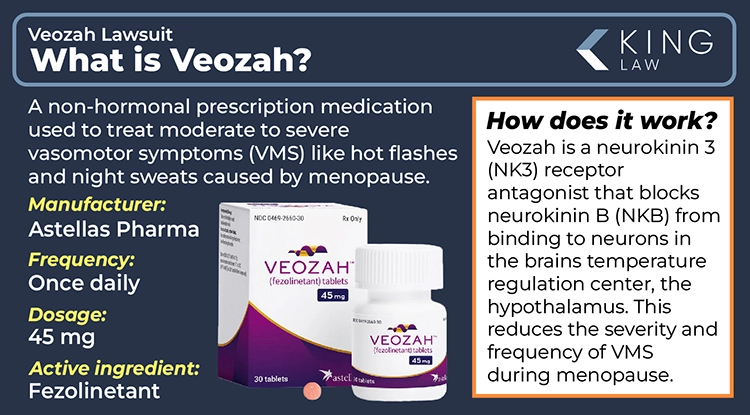
How Does Veozah Work?
Veozah (fezolinetant) is a neurokinin 3 (NK3) receptor antagonist that works by blocking neurokinin B (NKB) from binding to neurons in the hypothalamus, the brain’s temperature regulation center. This helps normalize activity in the brain’s thermoregulatory center, reducing the frequency and severity of vasomotor symptoms during menopause, like hot flashes. Clinical trials have shown significant symptom relief for most women.
During menopause, estrogen changes can cause excessive signaling in the hypothalamus, creating vasomotor symptoms (VMS) like hot flashes and night sweats. Veozah helps to regulate body temperature, reducing the frequency and severity of these symptoms. It was the first non-hormonal option for managing menopausal symptoms, often desired by women who can’t or won’t use hormone replacement therapies. Patients may begin showing symptom relief as early as one week after starting the medication. In clinical trials, 81-95% of women experienced a 50% or greater reduction in VMS frequency.
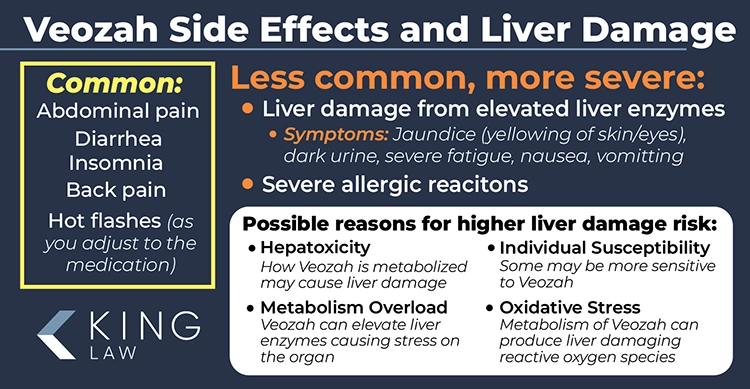
Veozah Side Effects
Common side effects of Veozah include abdominal pain, diarrhea, insomnia, and back pain. However, new research indicates that the medication may cause rare but serious side effects like liver problems and severe allergic reactions requiring constant monitoring and immediate medical attention. Symptoms that Veozah patients should watch for include jaundice, difficulty breathing, and dark urine.
Common Side Effects of Veozah:
- Abdominal pain
- Diarrhea
- Insomnia
- Back pain
- Hot flashes (as the body adjusts to the medication)
Less Common but Serious Side Effects of Veozah:
- Liver Problems: Symptoms include Jaundice (yellowing of the skin and eyes), dark urine, severe fatigue, nausea, vomiting, and light-colored stool. Monitoring liver function during treatment is recommended.
- Severe Allergic Reactions: Symptoms include breathing problems, racing heart, fever, swollen lymph nodes, swelling of the face or throat, hives, dizziness, and joint pain. Immediate medical attention required if symptoms occur.
Regular liver function tests are advised while taking the drug. Due to the risks associated with use, Veozah may not be suitable for individuals with pre-existing liver conditions.
Veozah and Liver Damage Due to Elevated Liver Enzymes
Veozah has been associated with rare but severe liver injury, including elevated liver enzyme levels (markers of liver stress). Patients taking the non-hormonal prescription medication should undergo liver function tests (LFTs) before starting the medication to check for pre-existing liver issues and regularly during treatment (monthly for the first 2 months, then at months 3, 6, and 9) to monitor liver health.
It is also recommended that patients stop taking Veozah and consult a healthcare provider if symptoms such as jaundice, dark urine, persistent fatigue, nausea, vomiting, pale feces, loss of appetite, weakness, unusual tiredness, or abdominal pain in the upper right quadrant occur.
Potential Reasons Veozah Patients May Be at Risk for Liver Damage:
- Hepatotoxicity: Veozah can cause liver cell damage due to how it is metabolized.
- Metabolism Overload: Stress on the liver from processing the drug can elevate liver enzymes.
- Individual Susceptibility: Some individuals are more sensitive to the drug, potentially leading to liver damage.
- Oxidative Stress: Metabolism of Veozah can produce reactive oxygen species (ROS) that damage liver cells.
If liver enzyme levels increase significantly or symptoms appear, Veozah should be discontinued, and a healthcare provider should be consulted. Veozah may not be suitable for individuals with pre-existing liver conditions or who are at a heightened risk for liver injury due to the risk of hepatotoxicity and oxidative stress.
Veozah Development, Approval, and Marketing Strategy
Developed by Astellas Pharma, Veozah began by undergoing preclinical research on neurokinin 3 receptors (NK3R) for vasomotor symptoms (VMS) in menopause before progressing through Phase 2 and Phase 3 clinical trials (SKYLIGHT 1 & 2) to confirm its safety and efficacy. Astellas is a global pharmaceutical company based in Tokyo, Japan, known for innovative medicines in oncology, urology, and immunology. It was founded in 2005 through the merger of Yamanouchi Pharmaceutical Co., Ltd. and Fujisawa Pharmaceutical Co., Ltd.
In the mid-2010s, the Phase 2 clinical trials focused on safety, dosage, and efficacy. During the Phase 3 (SKYLIGHT 1 & 2) trials, which concluded in 2022, Astellas aimed to confirm the drug’s safety and efficacy. The drug received FDA approval in May 2023 and became available in the U.S. by the middle of the year.
Marketing Strategy of Veozah:
- Direct-to-consumer: Targeted menopausal women through direct-to-consumer campaigns on television, online ads, and social media. Digital campaigns focused on educational content about menopause and non-hormonal treatments, including collaborations with influencers and advocacy groups.
- Emphasis on Non-Hormonal Nature: The manufacturer emphasized its non-hormonal nature, targeting women with contraindications or concerns about hormone replacement therapy (HRT). Promoted as a safer alternative to HRT which is linked to risks like cancer and cardiovascular issues.
- Physician Outreach Programs: Engaged in healthcare provider marketing, such as sales visits, product literature, and educational materials for gynecologists, endocrinologists, and primary care physicians. Also offered Continuing Medical Education (CME) courses through programs to educate providers about menopause, including Veozah.
- Educational Content: The manufacturer launched campaigns on VMS, menopause, and treatment options for both patients and healthcare providers.
Press releases and media coverage highlighted Veozah’s non-hormonal benefits and safety profile. Messaging was focused on Veozah’s avoidance of risks linked to hormone therapy. In September 2024, the FDA added a warning about the risk of liver injury to the existing warning about elevated function test values and required liver function testing in the original prescribing information.

Eligibility Criteria in the Veozah Lawsuit
Due to the widespread use of Veozah, many people are expected to be eligible to file a lawsuit. To qualify, individuals must have been prescribed Veozah for moderate to severe hot flashes, be able to provide proof of usage, and have been diagnosed with liver damage or a related condition like elevated liver enzymes or liver failure.
Eligibility Criteria in a Veozah Lawsuit:
- Use of Veozah: Must have been prescribed and taken Veozah (fezolinetant) for moderate to severe vasomotor symptoms (hot flashes).
- Proof of Use: Proof of Veozah usage (e.g., medical records, pharmacy receipts, or prescription documentation) is required.
- Liver Injury Diagnosis: Must have been diagnosed with liver damage or related conditions during or after Veozah use, such as elevated liver enzymes (AST, ALT), hepatitis, jaundice, fatty liver disease, and liver failure.
The severity of liver damage or harm and its impact on health and quality of life are usually key factors in the strength of the claim. Cases involving hospitalization, liver transplants, or permanent liver impairment may have stronger claims.

Evidence Needed to File a Veozah Lawsuit
Successful Veozah lawsuits generally require substantial evidence to substantiate the claim. Evidence such as medical records showing Veozah prescriptions and liver damage diagnosis, liver function test results, and expert medical testimony often prove critical in these cases. Working with an attorney to determine what evidence you will need to gather to help prove your case is essential.
Evidence Needed in a Veozah Lawsuit:
- Prescription records: Proof that you were prescribed and took Veozah (e.g., pharmacy receipts, medication logs).
- Liver injury diagnosis: Medical records confirming liver damage (e.g., elevated liver enzymes, hepatitis, liver failure).
- Liver function test (LFT) results: Tracking liver enzyme levels (AST, ALT) before and during Veozah use.
- Doctor’s notes: Assessments linking Veozah to liver damage.
- Use timeline: Document when you started and stopped Veozah, and when liver damage was diagnosed.
- Pre-existing conditions: Proof of normal liver function prior to using Veozah.
- Medical expert opinion: Testimony connecting Veozah to liver injury.
- Causality assessment: Evidence that rules out other causes of liver damage.
- Current medical costs: Bills for liver injury treatment (e.g., hospitalization, doctor visits).
- Future expenses: Estimated costs for ongoing liver treatment.
- Proof of lost income: Documentation of income loss (e.g., pay stubs, tax returns).
- Impact on future earnings: Evidence of reduced earning capacity due to long-term liver injury.
- Liver monitoring compliance: Documentation of regular liver function testing during Veozah use.
- Clinical trial involvement: If applicable, documentation of participation in Veozah clinical trials and any related liver function issues.
Recoverable Damages in the Veozah Lawsuit
Veozah patients diagnosed with liver injury may be eligible for past and future medical expenses, lost wages, as well as damages related to pain and suffering, diminished quality of life, permanent disability, disfigurement, punitive damages, loss of consortium, wrongful death (if applicable), and other out-of-pocket expenses. The severity of the liver injury, impact on life, and any negligence by Astellas Pharma may affect the overall value of the lawsuit.
Potential Damages in a Veozah Lawsuit:
- Past Medical Bills: Costs of liver injury treatment, including hospital stays, tests, medications, and surgeries.
- Future Medical Expenses: Compensation for ongoing treatment or monitoring, such as liver transplants, medications, or rehabilitation.
- Past Lost Wages: Compensation for income lost during recovery from the liver injury.
- Future Lost Earning Capacity: For long-term or permanent disability affecting the ability to work.
- Physical Pain: Compensation for discomfort from liver failure, surgery, or chronic conditions.
- Emotional Distress: Damages for anxiety, depression, or psychological trauma due to the injury.
- Loss of Quality of Life: Compensation for diminished ability to perform daily activities, hobbies, or enjoy life due to the liver injury.
- Permanent Disability: Compensation for long-term liver damage or transplant requirements.
- Disfigurement: Damages for physical disfigurement or scarring from treatment.
- Punitive Damages: Awarded if Astellas Pharma is found negligent or reckless in warning about liver injury risks.
- Loss of Consortium: Compensation for negative impacts on relationships with a spouse or partner due to the injury.
- Wrongful Death (if applicable): Compensation for families of deceased individuals, covering funeral expenses, loss of support, and emotional distress.
- Other Out-of-Pocket Expenses: Compensation for travel, home modifications, or other expenses incurred due to the liver injury.
- Legal Fees and Costs: Possible recovery of litigation costs, including attorney fees and court expenses.
Individuals who have suffered more severe or permanent liver damage may be entitled to higher compensation. Significant effects on work, quality of life, or family relationships may also increase potential compensation. Finally, proven misconduct by Astellas Pharma may result in punitive damages, raising the lawsuit’s value.
How to File a Veozah Lawsuit
Individuals who believe they may have a valid Veozah lawsuit should consult a pharmaceutical litigation attorney to evaluate their case. The attorney will help guide you through the legal process, including gathering relevant medical documentation (such as prescription records and liver damage diagnosis), ensuring the claim is filed within the statute of limitations, determining legal grounds (like product liability or negligence), and either negotiating a settlement or proceeding to trial, if necessary, to seek compensation for damages like medical expenses and lost wages.
Steps to File a Veozah Lawsuit:
- Consult an Attorney Specializing in Pharmaceutical Litigation: Find an experienced mass torts attorney to evaluate your case and determine eligibility. The lawyer will review your medical history, prescription details, and liver injury diagnosis.
- Gather Relevant Medical Documentation: Collect prescription records, medical records indicating liver damage, liver function test results, doctor’s notes, and hospital bills.
- Ensure You Are Within the Statute of Limitations: Ensure the lawsuit is filed within your state’s legal deadline, starting from when the injury was discovered.
- Determine Legal Grounds for the Lawsuit: Possible claims include product liability (drug defect), failure to warn (about liver risks), or negligence by Astellas Pharma.
- File the Lawsuit in Court: Your attorney will file the lawsuit, potentially as part of a multidistrict litigation (MDL) or class action.
- Pre-Trial Discovery: Both sides gather additional evidence, including medical expert testimony and depositions.
- Settlement Negotiations: Your attorney may negotiate with Astellas Pharma for a settlement before going to trial.
- Trial (If Necessary): If no settlement is reached, the case will go to trial, where evidence and arguments for compensation will be presented.
- Receive Compensation: If successful, compensation may include medical expenses, lost wages, pain and suffering, and potentially punitive damages. Compensation will generally depend on the severity of the injury and the outcome of the case, i.e., whether it was a negotiated settlement or went to trial.
It is imperative to remain in constant communication with the attorney you retain and to understand what to expect throughout the process. Litigation can be lengthy, but settlements may expedite resolution. In most cases, Veozah lawsuits are handled on a contingency fee basis, with no upfront fees.
Veozah Lawsuit Statute of Limitations
The statute of limitations for filing a Veozah lawsuit varies by state, generally ranging from 1 to 6 years for personal injury claims. It is crucial to consult a dangerous drugs lawyer as soon as possible to ensure the lawsuit is filed within the legal deadline to avoid losing the opportunity for compensation.
Statute of Limitations for Filing a Veozah Lawsuit:
- Time-Sensitive Filing: Lawsuits must be filed before the statute of limitations expires, as pursuing compensation after the deadline is typically not possible.
- State-Specific Deadlines: Statutes of limitations vary by state and claim type, ranging from 1 to 6 years for personal injury claims in the U.S.
- Legal Guidance: Contact a lawyer promptly to ensure your lawsuit is filed within the applicable time frame.
- Strict Deadlines: Acting quickly is essential due to the strict legal deadlines imposed by state laws.
Veozah Settlements and Payout Amounts
While specific settlement figures for Veozah liver injury lawsuits are not yet available, potential payouts will be influenced by factors such as the severity of the injury, medical expenses, lost wages, pain and suffering, and punitive damages. Based on similar pharmaceutical injury cases, estimates for Veozah settlements and payouts range from $50,000 for minor injuries to over $1 million for severe injuries or wrongful death.
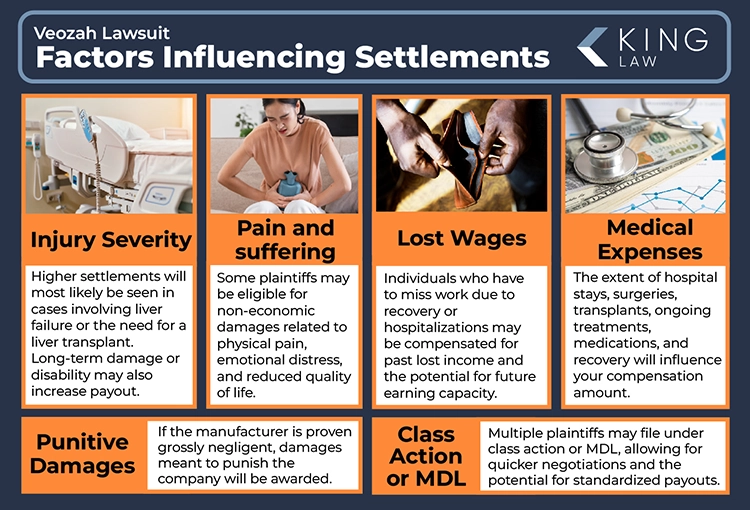
Factors Influencing Veozah Settlements:
- Severity of the Injury: Cases involving liver failure or the need for a liver transplant will likely result in higher settlements. Additionally, plaintiffs with long-term liver damage or disability may receive larger payouts.
- Medical Expenses: Extensive hospital stays, surgeries, or transplants will significantly increase the settlement amount. Costs for ongoing treatment, medications, and recovery also factor into higher compensation.
- Lost Wages and Future Earning Capacity: Individuals who have to miss work due to recovery may receive compensation for income lost. Settlements may also cover lost earning potential for plaintiffs who are permanently unable to work.
- Pain and Suffering: Some plaintiffs may be eligible for non-economic damages for physical pain, emotional distress, and reduced quality of life.
- Punitive Damages: If Astellas Pharma is proven to have acted grossly negligent, punitive damages may be awarded, increasing the payout.
- Class Action or Multidistrict Litigation (MDL): Multiple plaintiffs may file under class action or MDL, allowing for quicker settlement negotiations and potentially standardized payouts.
Settlements for comparable cases with other drug-related liver injuries, such as Tylenol or Acetaminophen cases, may be looked to for an estimated payout. These lawsuits generally ranged from $100,000 to several million dollars.
Estimated Payouts for Veozah Lawsuits:
- Minor Liver Damage: $50,000 – $200,000.
- Moderate Liver Injury: $200,000 – $500,000.
- Severe Liver Injury: $500,000 – $1 million+.
- Wrongful Death: Settlements typically exceed $1 million.
While no Veozah-specific settlements are available yet, plaintiffs should focus on gathering evidence and working with an experienced attorney.
Contact a Veozah Lawyer
Were you diagnosed with a liver injury after taking Veozah, you may be entitled to compensation. It is important to contact a lawyer as soon as possible. Critical factors to consider when choosing a Veozah attorney include experience, track record, and whether they offer a personalized approach. At King Law, our attorneys have extensive experience handling dangerous drug cases and will ensure you receive the effective representation you need and deserve. Contact our office today to schedule a free consultation.

