
Depo-Provera is a contraceptive injection that has been used by hundreds of thousands of women throughout the United States. Multiple lawsuits allege the drug’s active ingredient, medroxyprogesterone acetate (MPA), may increase a woman’s risk for developing brain tumors. These tumors are called intracranial meningiomas, and they can be very hazardous to a woman’s health. Further allegations include that the manufacturer of the drug, Pfizer, Inc., knew or should have known about the potential dangers associated with using the drug and failed to warn consumers.
We expect cases will be consolidated into multidistrict litigation (MDL) to help streamline the legal process. A class action lawsuit is also possible. Due to the widespread use of the contraceptive, significantly more lawsuits are expected to be filed in the near future. If you have been diagnosed with a brain tumor after taking Depo-Provera, you may be eligible for compensation and are encouraged to take legal action.
Depo-Provera Brain Tumor Lawsuit Overview
Depo-Provera is a contraceptive injection used to prevent pregnancy. It is a widely used, long-term birth control method containing the hormone MPA. Although it is highly effective at preventing pregnancy, research suggests it can cause brain tumors and other serious side effects. People who have been diagnosed with brain tumors after receiving Depo-Provera injections are filing lawsuits against the drug’s manufacturers, including Pfizer.
Legal documents filed against Pfizer and other manufacturers allege that the companies knew or should have known that patients were at an increased risk of developing meningiomas, particularly after prolonged Depo-Provera use. Symptoms of meningiomas include headaches, vision problems, seizures, and other neurological issues. These symptoms have the potential to cause severe disability, permanent impairment, or life-threatening conditions.
Primary Claims In Depo-Provera Lawsuits
Lawsuits filed against Depo-Provera manufacturers allege they knew their drug could cause brain tumors, cancers, and bone loss and yet they failed to warn doctors and patients about these risks. Additionally, Pfizer and other defendants are accused of overlooking or downplaying adverse event reports. Lawsuits say the companies failed to make timely updates to the Depo-Provera’s label and properly warn people about the risks of these conditions. Instead, the lawsuits say manufacturers ignored growing evidence, prioritizing profits and market share over patient safety. Depo-Provera patients who develop brain tumors are encouraged to seek legal counsel for assistance with filing a claim.

Depo-Provera Lawsuit – 2025 Update
November 7, 2024: Depo-Provera Settlements and Awards Could Have High Dollar Values
November 1, 2024: Depo-Provera Lawsuits Focus on Meningiomas, Potential Breast Cancer Link
November 1, 2024: Another Woman Battling Brain Tumors Files a Depo-Provera Lawsuit Against Pfizer and Other Defendants
A woman in California has filed a Depo-Provera lawsuit after being diagnosed with an intracranial meningioma. Anjanna Lawson began receiving Depo-Provera injections at the age of 16 and continued to receive them through September 2024. In July of 2023, Lawson was diagnosed with an intracranial meningioma and underwent a right pterional craniotomy to remove the tumor. However, in early 2024, a residual tumor was discovered, and Lawson had five sessions of stereotactic radiosurgery. Her tumor has still not been eradicated and she endures vision loss, facial spasms, and a burning sensation in her face. In the complaint filed as part of her Depo-Provera lawsuit, she is seeking damages from Pfizer, Inc., Viatris, Inc., Greenstone, LLC, Prasco, LLC, and Pharmacia & Upjohn LLC, and Pharmacia, LLC.
October 29, 2024: New Depo-Provera Lawsuit Filed in California with Potential for Multidistrict Consolidation
Another Depo-Provera lawsuit has been filed. Kathleen Fazio v. Pfizer, Viatris, Inc., Greenstone, LLC, Prasco Labs, Pharmacia, and Upjohn was filed on October 28, 2024, in the Central District of California. The five initial court filings in federal court suggest that plaintiff lawyers will soon request the Judicial Panel on Multidistrict Litigation to consolidate the Depo-Provera lawsuits. Consolidation means that the discovery phase for each Depo-Provera case would be handled by a group of lawyers appointed by a single federal court judge.
It is noteworthy that no lawsuits have been filed in New York federal court. Pfizer is based in New York and would be a logical place for Depo-Provera lawsuits to be filed. However, plaintiff lawyers have avoided filing in New York due to prior unfavorable rulings in federal cases. The plaintiff lawyers are carefully selecting jurisdictions where lawsuits will be filed. We anticipate early cases will be filed in California, Massachusetts, and potentially Vermont and Illinois. These four states implicitly or explicitly accept a legal theory known as innovator liability, which is expected to be significant in claims brought against Pfizer by users of generic Depo-Provera.
October 28, 2024: Californian Woman Files Depo-Provera Lawsuit As Cases Against Pfizer Increase
A Central California woman has filed another Depo-Provera lawsuit in the Central District of California. The lawsuit will be referred to as Monique Jones v. Pfizer, et. al. We are beginning to see a trend of where cases are being filed, with two cases filed in the Northern District of California and one in the Southern District of Indiana. We suspect this trend to continue because Indiana and California have strong state laws on innovator liability. Innovator liability is a legal concept where the innovator (the original manufacturer) can be held liable for alleged injuries inflicted by generic drugs. Generic drugs must mirror the exact language of the original warning label. So, if a person takes a generic drug, the originator (innovator) of the drug can be liable because they intentionally or negligently withheld information about the safety of the drug. This concept is included in the lawsuit filed by Jones and her legal team.
October 25, 2024: Second Depo-Provera Lawsuit Filed Against Pfizer by Indiana Woman
On October 15, a woman in Indiana filed a lawsuit against Pfizer claiming her brain tumor and subsequent injuries were caused by Depo-Provera use. Plaintiff Lesley Noble received Depo-Provera injections over the course of 20 years. Noble’s intracranial meningioma was discovered in 2017. She underwent invasive surgery to remove her brain tumor, but after 6 months, her tumor aggressively regrew. She underwent 36 rounds of radiation during her second treatment, all while she was still taking Depo-Provera, not knowing it likely caused her tumors. Lesley Noble and her Husband Justin Noble are demanding a jury trial for the many injuries Lesley has endured.
October 22, 2024: Pfizer and Co-Defendants Face 9 Accusations In First Depo-Provera Lawsuit
Pfizer, Inc. and its co-defendants, Viatris, Inc., Greenstone LLC, Prasco Labs, and Pharmacia & Upjohn, face nine allegations of wrongdoing in a federal Depo-Provera lawsuit. Kristina Schmidt filed a lawsuit in California Federal Courts that claims her brain tumor was caused by years of Depo-Provera use. In that lawsuit, Schmidt’s lawyers list nine allegations against the defendants, including Failure to Warn, Design Defect, Negligence, Negligent Failure to Warn, Negligent Design Defect, Negligent Design Defect, Negligent Misrepresentation, Fraudulent Misrepresentation, Breach of Express Warranty, and Breach of Implied Warranty. The charges accuse Pfizer and the other defendants of knowingly designing, marketing, and manufacturing a drug that could cause intracranial meningiomas without properly warning physicians or patients.
October 17, 2024: Evidence Suggests Exposure to Hormones in Depo-Provera May Cause Spinal Tumors
We are investigating Depo-Provera cases that involve meningiomas of the spinal cord. Recent studies have linked the use of Depo-Provera to brain tumors, specifically intracranial meningiomas. New research is focusing on meningiomas that form on the spinal cord. An article published in the journal of Neuro-Oncology Advances adds that while less than 13% of meningiomas are on the spinal cord, meningiomas make up about a quarter of the tumors on the spinal cord. According to the article, external hormone sources, such as injections like Depo-Provera, may be linked to a higher risk of developing meningiomas in the brain or spinal cord.
October 11, 2024: Pfizer’s Legal Battle Over Depo-Provera and Generic Drug Liability
One of the central issues in the Depo-Provera lawsuit is whether Pfizer is legally responsible for meningiomas and brain tumors caused by “generic” versions of Depo-Provera. In Mutual Pharmaceutical Co. v. Bartlett, the United States Supreme Court ruled that manufacturers of generic drugs are not legally responsible if the drug they make has the same active ingredient as an approved drug. However, in the Depo-Provera case, the “generic” manufacturers are mostly wholly owned subsidiaries of Pfizer.
Additionally, the “generic” drugs mentioned in the Depo-Provera lawsuits are exact copies of Depo-Provera and were manufactured at the same sites as Depo-Provera, in locations owned by Pfizer. For example, the complaint in the recently filed case of Schmidt v. Pfizer also names Greenstone LLC as a defendant. Greenstone LLC was owned by Pfizer but now operates under the Viatris umbrella.
We expect questions about whether Pfizer is legally responsible for meningiomas caused by “authorized generics” to be aggressively litigated for several years as part of this lawsuit. Pfizer has owned the rights to Depo-Provera since 2002, when they purchased Upjohn.
October 4, 2024: Woman Files Lawsuit Against Pfizer Alleging Depo-Provera Caused Her Brain Tumor
A woman has filed a lawsuit after taking Depo-Provera and developing an intracranial meningioma, a type of brain tumor. Kristina Schmidt filed her case against Pfizer in the United States District Court of the Northern District of California. Her attorneys allege Depo-Provera substantially contributed to the development of her brain tumor 17 years after taking the contraceptive. Since her diagnosis, she has endured significant, invasive treatments and experienced serious injuries. In the complaint filed in California Federal Court, her lawyers say that Pfizer, “knew or should have known for decades that Depo-Provera, when administered and prescribed as intended, can cause or substantially contribute to the development of meningiomas.” This case is part of a growing number of lawsuits against Pfizer for their injectable contraceptive.
October 4, 2024: Depo-Provera Tied to Highest Cancer Rate Increase Among Commercial Drugs
September 30, 2024: Depo-Provera Lawsuit Expands as More Plaintiffs Learn of Potential Risks
The Depo-Provera lawsuit is gaining traction as more possible plaintiffs become aware of the allegations against Pfizer, the birth control manufacturer. A French study showed a link between the Depo-Provera shot and meningiomas, a tumor that arises from the protective membranes covering the brain and spinal cord. Symptoms can include headaches, vision changes, seizures, memory loss, and dizziness. Further research must be conducted to understand why Depo-Provera may increase the risk of tumor growth. With 74 million women worldwide having used the drug, the potential scale of this litigation is significant.
September 25, 2024: Depo-Provera Linked to Brain Tumors, Lawsuits Expected to Rise
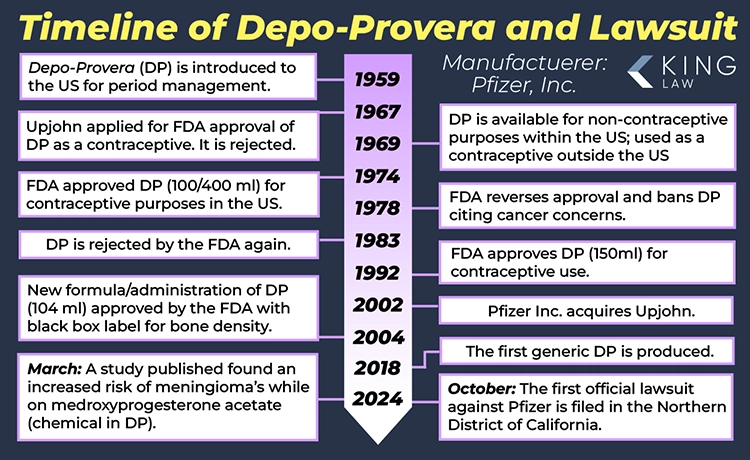
Depo-Provera has been around for a long time—a really long time. Originally developed in 1959, the drug was approved by the FDA in 1974, only to have its approval withdrawn in 1978 due to concerns it could cause cancer. It was reapproved by the FDA as a contraceptive in 1992. With indications it could cause cancer more than 45 years ago, it was no surprise when the British Medical Journal discovered a connection between Depo-Provera and the development of meningiomas and other brain tumors. More studies are forthcoming, and with the sheer volume of women who have taken this drug over the past five decades, a large number of lawsuits are expected in the future.
September 17, 2024: Pfizer Faces Renewed Legal Challenges Over Depo-Provera and Brain Tumor Risk
Pfizer is again facing legal pressure over its drug Depo-Provera. Recent news on Depo-Provera followed a study showing that a particular type of brain tumor, called meningiomas, is more than five times more likely in women who took the drug as few as two times. There were previous lawsuits alleging that Depo-Provera led to bone density loss. One notable case was Cassandra Colville v. Pharmacia & Upjohn, filed on July 10, 2008. Since then, the drug and the rights to manufacture and sell Depo-Provera have been sold to pharmaceutical giant Pfizer.
September 13, 2024: Study Highlights the High Value of Meningioma Lawsuits Related to Depo-Provera
September 10, 2024: Anticipated Federal Court Consolidation for Depo-Provera Claims Involving Brain Tumors
According to the CDC, approximately 24.5% of sexually active women have used the injectable contraceptive Depo-Provera at some point in their lifetime. Due to the drug’s widespread use, several women diagnosed with brain tumors are now filing claims against its manufacturers. While there are no significant developments in the Depo-Provera lawsuit at this time, our firm anticipates that these cases will ultimately be consolidated in federal court, which will initiate legal action against the drugmakers.
September 5, 2024: Depo-Provera’s History of FDA Rejections, Bone Density Concerns and Links to Brain Tumors
Depo-Provera was repeatedly denied approval by the FDA in the 1970s and 1980s, until it was finally approved in 1992. Since then, reports have suggested that the drug leads to reduced bone density and can cause significant side effects when women attempt to stop using it. Originally manufactured by Upjohn, Depo-Provera is now produced by Pfizer. Recent studies have also linked the drug to brain tumors.
August 2024: Depo-Provera cases continue to be filed nationwide. Affected individuals are encouraged to contact King Law to schedule a free case review.
March 2024: A study published in the British Medical Journal (BMJ) finds that people using an injectable form of medroxyprogesterone acetate for a year or more may have a higher risk of meningioma.
May 2008: The Superior Court of Quebec certified an action against Pfizer regarding Depo-Provera on behalf of a national class.
November 2004: The U.S. Food and Drug Administration (FDA) required a “black box” warning on the Depo-Provera label. The warning notes that prolonged use of the drug may cause irreversible and significant loss of bone density.
About the Depo-Provera Lawsuit:
New Research Links Depo-Provera to Brain Tumors in Women
Depo-Provera Manufacturer: Pfizer
Federal Lawsuit Filed Against Pfizer for Harms Caused by Depo-Provera
Eligibility Criteria in the Depo-Provera Lawsuit
Evidence Needed to File a Depo-Provera Lawsuit
Recoverable Damages in the Depo-Provera Lawsuit
How to File a Depo-Provera Lawsuit
Depo-Provera Lawsuit Settlement and Payout Amounts
What Is Depo-Provera?
Depo-Provera is a brand name for medroxyprogesterone acetate, a synthetic form of the hormone progestin used primarily as a contraceptive injection that prevents pregnancy by inhibiting ovulation, thickening cervical mucus, and thinning the uterine lining. It is injected into the arm or buttocks and administered every 12 weeks.
The medication, also known as the birth control shot or Depo-Provera shot, has a high rate of effectiveness and may also be prescribed for managing conditions like endometriosis, heavy menstrual bleeding, and reducing the risk of endometrial cancer. It can prevent pregnancy for up to 14 weeks if administered correctly and on schedule.

How Does Depo-Provera Work?
Depo-Provera works through several mechanisms, including preventing ovulation, thickening cervical mucus, and thinning the cervical mucus, ultimately creating an environment that is less than favorable for sperm survival and function.
How Depo-Provera works:
- Inhibition of Ovulation: Depo-Provera prevents the ovaries from releasing an egg (ovulation), eliminating the possibility of fertilization.
- Thickening of Cervical Mucus: The injection thickens cervical mucus, making it difficult for sperm to pass through the cervix and reach an egg.
- Thinning of the Uterine Lining: Depo-Provera thins the uterine lining, reducing the likelihood that a fertilized egg could implant and develop.
- Effect on Sperm: The hormone in Depo-Provera creates an environment that is less favorable for sperm survival and function, further preventing pregnancy.
When administered correctly on schedule, Depo-Provera is a highly effective contraceptive method. It is injected into a muscle, generally the arm or buttocks, every 12 weeks (approximately every 3 months). Allegations against the manufacturer of Depo-Provera include that prolonged use of the medication may put patients at an increased risk for developing brain tumors and other severe conditions.
Common Uses of Depo-Provera
Depo-Provera has a number of uses but is primarily prescribed as a form of long-acting, reversible contraceptive. It is also used to treat menstrual disorders, endometriosis, and hormonal imbalances.
Uses of Depo-Provera:
- Contraception: Primarily used as a long-acting reversible contraceptive, administered via injection every 12 weeks. It is highly effective in preventing pregnancy when used correctly.
- Management of Endometriosis: May be prescribed to reduce the growth of endometrial-like tissue outside the uterus. It can help alleviate pain and symptoms associated with endometriosis.
- Treatment of Menstrual Disorders: It is also used to manage heavy menstrual bleeding (menorrhagia) and painful periods (dysmenorrhea) due to its ability to thin the uterine lining and suppress ovulation, reducing bleeding and menstrual cramps.
- Reduction of Endometrial Cancer Risk: The drug may help lower the risk of endometrial cancer in women with certain risk factors, such as a history of endometrial hyperplasia.
- Management of Hormonal Imbalances: Has been prescribed to treat hormonal imbalances that cause irregular or absent menstrual periods (amenorrhea).
- Support in the Treatment of Uterine Fibroids: This medication helps manage symptoms associated with uterine fibroids, such as heavy bleeding, though it is not a cure for fibroids.
- Postpartum Bleeding Control: This can be used to control bleeding after childbirth.

Depo-Provera Side Effects
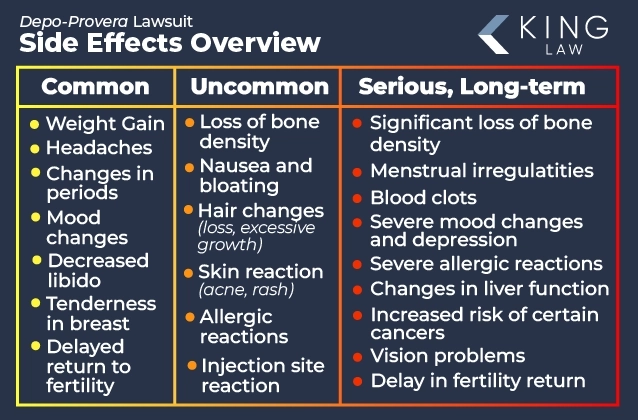
Depo-Provera may cause a range of side effects, including changes in menstrual periods, weight gain, headaches, and decreased libido. The injectable contraceptive is also associated with less common but serious side effects such as bone density loss, nausea, hair changes, and skin reactions. While some side effects diminish over time, others may be irreversible. It is essential to seek regular health check-ups to receive a prompt diagnosis and treatment of any adverse condition.
Common Side Effects of Depo-Provera:
- Changes in Menstrual Periods: Irregular bleeding or spotting, especially in the first few months. Periods may become heavier, lighter, or stop altogether.
- Weight Gain: Weight gain is a frequently reported side effect.
- Headaches: Headaches, including migraines, can occur.
- Mood Changes: Some users report mood swings, depression, anxiety, or nervousness.
- Decreased Libido: A reduction in sexual desire is reported by some women.
- Breast Tenderness: Tenderness or discomfort in the breasts may occur.
- Delayed Return to Fertility: Fertility may take several months to over a year to return after discontinuing Depo-Provera.
Less Common Side Effects of Depo-Provera:
- Bone Density Loss: Long-term use can lead to decreased bone mineral density, increasing the risk of osteoporosis and fractures; limiting use to 2 years is recommended unless additional use is necessary.
- Nausea and Bloating: Some women may experience nausea or bloating.
- Hair Changes: Hair loss or excessive hair growth can occur on the face or body.
- Skin Reactions: Acne, skin rash, or other skin reactions may develop.
- Allergic Reactions: Rare allergic reactions may include hives, itching, or swelling.
- Injection Site Reactions: Pain, swelling, or redness at the injection site.
Long-Term and Serious Side Effects
Long-term use of Depo-Provera can lead to significant health conditions, including irreversible bone density loss, a delayed return to fertility, and ongoing menstrual irregularities. Other potentially life-threatening side effects associated with the use of the medication include an increased risk of certain cancers, blood clots, and severe depression.
Long-Term Side Effects of Depo-Provera:
- Bone Density Loss: Due to estrogen suppression, Depo-Provera can lead to significant bone mineral density loss. There is also an increased risk of osteoporosis and fractures, especially with use beyond two years. For this reason, the medication is often recommended only for short-term use.
- Delayed Return to Fertility: Fertility may take several months to over a year to return after discontinuing Depo-Provera. This delay can be concerning for those wishing to conceive shortly after stopping the injection.
- Menstrual Irregularities: Long-term use can result in ongoing menstrual irregularities, including irregular bleeding, amenorrhea, or unpredictable periods.
Serious Side Effects Associated with Depo-Provera:
- Blood Clots: There is a rare but serious risk of developing blood clots, which can lead to conditions like deep vein thrombosis, pulmonary embolism, stroke, or heart attack.
- Severe Depression and Mood Changes: Some users may experience severe depression or significant mood changes, particularly those with a history of depression.
- Allergic Reactions: Rare cases of serious allergic reactions, including anaphylaxis, which require immediate medical attention.
- Liver Function Changes: Potential for liver function issues, including jaundice or other liver-related problems, particularly in those with a history of liver disease.
- Increased Risk of Certain Cancers: Limited evidence suggests a slightly increased risk of breast cancer with long-term use, though data is not conclusive. Women with a strong family history of breast cancer should discuss this risk with their healthcare provider.
- Vision Problems: Possible vision changes that could indicate a serious condition, like a blood clot in the eye, requiring immediate evaluation.
The FDA advises against using Depo-Provera for more than two years due to these serious risks, particularly bone loss. Patients are encouraged to contact their healthcare provider to discuss any adverse conditions developed after using the medication.
New Research Links Depo-Provera to Brain Tumors in Women
A 2024 study published in the British Medical Journal found that using drugs like Depo-Provera with the active ingredient medroxyprogesterone acetate for more than a year may increase the risk of intracranial meningioma by 5.6x, based on data from 18,061 women who underwent surgery for these typically benign brain tumors, which develop in the meninges. Meningiomas are generally benign (non-cancerous) tumors that develop in the meninges, the membranes covering the brain and spinal cord.
The study notes that the link between progestin-based contraceptives like Depo-Provera and an increased risk of meningiomas, particularly with high doses or prolonged use, may be driven by the hormone progestin’s ability to promote tumor growth through progesterone receptors. The risk of developing meningiomas appears to be related to the dose and duration of progestin exposure.
In some cases, discontinuing progestin-based therapy like Depo-Provera has been associated with stabilization or even regression of meningiomas, suggesting a potential reversibility of tumor growth. Although meningiomas are usually not life-threatening, surgery to remove them can be risky.

Who Are Women Filing Depo-Provera Lawsuits Against?
Women who developed intracranial meningiomas after Depo-Provera use are filing lawsuits against the manufacturers of Depo-Provera and its authorized generics. Defendants named in Depo-Provera Lawsuit include:
- Pfizer, Inc.
- Viatris Inc.
- Greenstone LLC
- Prasco, LLC d/b/a Prasco Labs
- Pharmacia & Upjohn Co, LLC
- Pharmacia LLC
Although the primary defendant in Depo-Provera lawsuits is Pfizer, other defendants are listed. Several lawsuits argue that these other defendants:
- Were either owned or acquired by Pfizer at the times they were manufacturing Depo-Provera
- Were making a generic equivalent at a Pfizer owned/operated facility
- Were producing a generic equivalent with the exact formulation of Pfizer’s brand-name Depo-Provera
Many of the existing lawsuits argue that Pfizer has been directly involved in manufacturing brand-name and generic equivalent Depo-Provera since 1993. Therefore, these lawsuits say Pfizer should be held liable for harming women.
Defendant and Depo-Provera Manufacturer: Pfizer
Pfizer, Inc., has been the manufacturer, marketer, and distributor of Depo-Provera since acquiring the rights through a merger with Pharmacia in 2003. The company is responsible for ensuring the drug’s global safety, efficacy, and quality standards. Under Pfizer’s ownership, Depo-Provera has become a widely used contraceptive with different formulations, including the standard Depo-Provera Contraceptive Injection and the lower-dose Depo-SubQ Provera 104.
However, the company has faced various lawsuits related to Depo-Provera, including claims concerning side effects like bone density loss and potential links to conditions such as meningiomas. The company must manage these legal issues while continuing to market and distribute Depo-Provera.
Federal Lawsuit Filed Against Pfizer for Harms Caused by Depo-Provera
On October 1, 2024, a California woman filed a lawsuit against Pfizer after taking Depo-Provera and developing a brain tumor. Kristina Schmidt used Depo-Provera for about 17 years and was diagnosed with a brain tumor at the age of 37.
Schmidt’s attorneys allege that her specific tumor, an intracranial meningioma, has been linked to progesterone-based contraceptives like Depo-Provera. Despite the known link, Schmidt’s attorneys say Pfizer failed to update Depo-Provera’s drug label to warn patients about the possible connection between the drug and intracranial meningiomas.
Accusations Made Against Pfizer in the Schmidt Lawsuit
According to the complaint filed by Schmidt’s lawyers, Pfizer should have known that Depo-Provera increases the risk of brain tumors. However, they have not disclosed this information to patients or doctors. The complaint states, “The association between progesterone and meningioma has been known or knowable for decades, particularly for sophisticated pharmaceutical corporations like Defendants engaging in FDA-required post-market surveillance of their products for potential safety issues. That duty includes an obligation to keep current with emerging relevant literature and where appropriate, perform their own long-term studies and follow-up research.”
Said more simply, Depo-Provera’s manufacturer (Pfizer) is required by the FDA to monitor the outcomes of people who use Depo-Provera. Schmidt’s lawyers accuse Pfizer of failing to perform the following actions:
- Monitoring for new side effects, especially ones that don’t happen immediately
- Conducting medical studies that examine the safety of Depo-Provera
- Closely following scientific literature that explored safety concerns about Depo-Provera
Because Pfizer did not take these actions or warn patients about the potential dangers of Depo-Provera, Schmidt’s lawyers are seeking damages to offset the pain, suffering, and losses she endured. Other plaintiffs will file lawsuits citing similar circumstances and seeking similar damages.
Eligibility Criteria in the Depo-Provera Lawsuit
In order to qualify for a Depo-Provera lawsuit, individuals must have used the brand-name (Pfizer) Depo-Provera, depo-SubQ Provera, or an authorized generic version at least twice and must have been diagnosed with a meningioma or brain tumor after usage. Eligibility may be further influenced by the duration of use, time elapsed since last use, and specific diagnosis, potentially entitling them to file a lawsuit against the manufacturer for compensation.
Eligibility criteria for a Depo-Provera lawsuit:
- Usage: Must have used brand-name Depo-Provera (Pfizer), brand-name depo-SubQ Provera (Pfizer), or an “authorized generic” version of Depo-Provera at least twice.
- Diagnosis: Diagnosed with meningioma or a brain tumor after using Depo-Provera.
- Other Considerations: The duration of Depo-Provera or its generic version usage, the time elapsed between the last use of Depo-Provera, the diagnosis, and the diagnosis of meningioma and brain tumors.
Due to the potential link between Depo-Provera and an increased risk of brain tumors in some women, individuals who have suffered a brain tumor after taking the medication may be entitled to file a lawsuit against the manufacturer.
Exclusions: Disqualifying Factors in the Lawsuit
Not all individuals taking an injectable contraceptive will qualify for a Depo-Provera lawsuit. For instance, patients who have only used non-brand or unauthorized generic versions, have insufficient usage of the drug, were diagnosed with a brain tumor before using Depo-Provera, or have pre-existing neurological conditions may not qualify for compensation.
Exclusions for Depo-Provera Lawsuit Eligibility:
- Non-Brand or Unauthorized Generic Use: Individuals who have only used non-brand (non-Pfizer) or unauthorized generic versions of Depo-Provera or depo-SubQ Provera may not qualify.
- Insufficient Usage: Individuals who have used Depo-Provera, depo-SubQ Provera, or an authorized generic version fewer than two times may be excluded.
- Diagnosis Before Usage: Individuals diagnosed with meningioma or a brain tumor prior to using Depo-Provera, depo-SubQ Provera, or an authorized generic may not be eligible.
- No Diagnosed Brain Tumor: Individuals who have not been diagnosed with meningioma or any brain tumor after using Depo-Provera may not qualify, regardless of other symptoms.
- Use of Other Contraceptives: Individuals whose brain tumor diagnosis could be attributed to other hormonal contraceptives or medications, rather than Depo-Provera or its authorized generic, may be excluded.
- Pre-Existing Conditions: Individuals with a history of brain tumors, meningiomas, or other neurological conditions predating their use of Depo-Provera may not qualify.
- Alternative Diagnoses: Individuals diagnosed with brain tumors or neurological conditions not related to progestin or Depo-Provera may be excluded.
- Timeframe Restrictions: There may be a time limit on how long after the last use of Depo-Provera a diagnosis must be made to qualify. Diagnoses made beyond this timeframe may result in exclusion.

Evidence Needed to File a Depo-Provera Lawsuit
It is vital to gather the necessary evidence in order to substantiate your claim. Evidence that may help support your case includes comprehensive medical records, prescription records, personal and witness testimony, financial records proving damages, and proof that the product used was the Pfizer-manufactured Depo-Provera or an authorized generic.
Evidence Necessary for a Depo-Provera Lawsuit:
- Documentation of Depo-Provera Use: Medical records, including prescription records and doctor’s notes, showing that the plaintiff received Depo-Provera, depo-SubQ Provera, or an authorized generic at least twice.
- Diagnosis Records: Proof of diagnosis for a meningioma or brain tumor, with pathology reports, MRI or CT scan results, and neurologist or oncologist records showing the diagnosis occurred after starting Depo-Provera.
- Treatment Records: Documentation of treatments received for the tumor, such as surgery, radiation therapy, or medication.
- Pharmacy and Prescription Records: Detailed records showing the dispensing of Depo-Provera or its authorized generic, establishing the timeline and frequency of use.
- Plaintiff’s Testimony: A personal account of Depo-Provera use, the onset of symptoms, and the impact of the tumor on their life, including pain, suffering, and loss of income.
- Witness Testimony: Statements from family, friends, or coworkers corroborating the impact on the plaintiff’s life.
- Financial Records: Documentation of economic losses due to medical bills, lost wages, and other expenses related to the tumor.
- Impact on Quality of Life: Evidence showing how the diagnosis affected the plaintiff’s quality of life, including work incapacity, loss of enjoyment, or psychological trauma.
- Timeline of Events: A clear timeline linking the start of Depo-Provera use, the tumor’s diagnosis, and the condition’s progression.
- Verification of Pfizer’s Involvement: Evidence that the Depo-Provera used was the brand-name product manufactured by Pfizer, or an authorized generic is crucial for establishing the defendant in the lawsuit.
Specific Pieces of Evidence to Collect:
- Hospital bills related to treatment for the brain tumor or meningioma.
- Prescription records for Depo-Provera, depo-SubQ Provera, or authorized generics.
- Doctor’s notes detailing the prescription and administration of Depo-Provera.
- Diagnosis of meningioma or brain tumor, including medical reports.
- Imaging results (MRIs, CT scans, etc.) confirming the presence of the brain tumor or meningioma.
- Pathology reports providing details on the type and characteristics of the tumor.
- Treatment records, including surgery, radiation therapy, or medication details.
- Pharmacy receipts for Depo-Provera or depo-SubQ Provera purchases.
- Dispensing records showing the dates and dosages of Depo-Provera or its authorized generic.
- Prescription refill history from pharmacies or healthcare providers.
- Detailed medical bills and receipts for treatments, hospital stays, and medications.
- Pay stubs or employment records showing lost wages due to illness or treatment.
- Receipts for out-of-pocket expenses related to the brain tumor treatment or care.
- Written statements from the plaintiff detailing their experience with Depo-Provera and the onset of symptoms.
- Testimonies from family members, friends, or coworkers about the impact of the condition on the plaintiff’s life.
- Notes or records from therapy or counseling sessions related to the psychological effects of the diagnosis.
Recoverable Damages in the Depo-Provera Lawsuit
Depo-Provera patients diagnosed with a brain tumor may be entitled to recover a range of damages, including past and future medical expenses, compensation for lost wages and diminished earning capacity, damages for physical pain, emotional suffering, and loss of enjoyment of life.
Damages in a Depo-Provera Lawsuit:
- Past Medical Expenses: Costs for diagnosis, treatment, surgery, hospitalization, and medications up to the present.
- Future Medical Expenses: Projected costs for ongoing or future care, including surgeries, rehabilitation, long-term care, and medications.
- Past Lost Wages: Compensation for income lost due to inability to work during treatment or recovery.
- Future Lost Earnings: Compensation for reduced earning potential due to lasting effects of the condition.
- Loss of Earning Capacity: Damages for diminished future earning potential as a result of the condition.
- Physical Pain: Compensation for the physical pain endured due to the tumor and its treatment.
- Emotional and Mental Suffering: Compensation for emotional distress, anxiety, depression, and mental anguish caused by the condition.
- Diminished Quality of Life: Compensation for the loss of enjoyment in activities, hobbies, and daily living.
- Permanent Disability or Disfigurement: Damages for any permanent impairment resulting from the tumor or its treatment and compensation for permanent physical disfigurement caused by surgery.
- Loss of Consortium: Compensation for the impact on the plaintiff’s relationship with their spouse, including loss of companionship and marital relations.
- Punitive Damages: Awarded in cases of particularly reckless or malicious conduct by the defendant, meant to punish and deter similar actions.
- Out-of-Pocket Costs: Reimbursement for additional costs directly related to the tumor, such as transportation, home care, or special equipment.
- Legal Fees and Court Costs: Potential recovery of legal representation costs (attorneys fees) and reimbursement for filing fees, expert witness fees, and other lawsuit-related expenses.
Individuals who have lost a loved one as a result of Depo-Provera use may be entitled to a wrongful death claim. Recovery for a wrongful death lawsuit may include compensation for costs related to the plaintiff’s death, such as funeral and burial services, compensation for the financial support the deceased would have provided (loss of support), and damages for the loss of the deceased’s companionship and emotional support to family members (loss of companionship).
How to File a Depo-Provera Lawsuit
To file a Depo-Provera lawsuit, it is critical to follow specific steps and procedures, including confirming eligibility and gathering necessary evidence to prove your case. Consulting with an experienced attorney as early in the process as possible can help ensure the highest probability of success.
Steps to File a Depo-Provera Lawsuit:
- Confirm Eligibility: Ensure you have a confirmed diagnosis of meningioma or another type of brain tumor after using Depo-Provera. Prior to filing, it is important to collect proof of your Depo-Provera use, such as medical records, pharmacy receipts, or prescription history that shows you received the injection at least twice.
- Consult a Highly-Qualified Lawyer: Seek out attorneys who are well-versed in pharmaceutical litigation or product liability, particularly those with experience in Depo-Provera or hormone-related cases. Many law firms offer free consultations to determine the strength of your case and discuss potential legal strategies.
- Gather and Prepare Necessary Documentation: Collect all relevant medical records that confirm your brain tumor diagnosis, including imaging results (MRIs, CT scans) and treatment history; gather tangible evidence of your Depo-Provera usage, such as prescription records, pharmacy receipts, and packaging labels; and compile statements from healthcare providers, family members, or friends who can corroborate your Depo-Provera use and the onset of your symptoms.
- File the Lawsuit: Your attorney will draft and file a legal complaint against Pfizer, the manufacturer of Depo-Provera, in the appropriate court. They will also help to ensure the lawsuit is filed within the legal time limits, which vary by state but typically range from one to several years from the date of diagnosis or discovery of the tumor link.
- Discovery Phase: During the Discovery Phase, both sides will exchange relevant information and evidence, including medical records, expert testimonies, and depositions. Both parties may hire medical and pharmacological experts to testify about the link between Depo-Provera and brain tumors.
- Settlement Negotiations: Before trial, there is often an attempt to settle the case out of court. Your lawyer will negotiate with Pfizer’s representatives to secure fair compensation. Reviewing all settlement offers with your attorney to determine if they sufficiently cover medical expenses, lost income, pain and suffering, and other damages is essential.
- Trial: If a settlement cannot be reached, your case may proceed to trial, where your attorney will present your evidence to a judge or jury. Both sides will present their arguments and evidence, and a verdict will be made based on the case’s merits.
- Post-Trial: If the trial outcome is unfavorable, you may have the option to appeal the decision. If you win the case, steps will be taken to collect the awarded damages.
- Monitor Health and Follow-Up: Continue to monitor your health and receive ongoing medical care as needed for your condition. Maintain communication with your lawyer for any post-trial follow-ups or to manage the appeals process if necessary.
Depo-Provera Lawsuit Settlement and Payout Amounts
Settlement amounts in Depo-Provera lawsuits can vary widely depending on factors such as the severity of the condition, medical expenses, lost wages, pain and suffering, and the potential for punitive damages. While payout amounts are speculative due to the early stages of litigation, typical settlements are expected to range from $100,000 to $5 million or more, depending on the specifics of each case.
Factors Influencing Depo-Provera Lawsuit Settlements:
- Severity of the Condition: The extent, size, location, and impact of the brain tumor or meningioma on the plaintiff’s health and daily life significantly influence settlement amounts. Cases involving severe, life-threatening tumors or those causing permanent disability tend to result in higher payouts.
- Medical Expenses: Costs for past and future medical treatments, including surgeries, hospital stays, medications, ongoing care, and rehabilitation, are critical factors. Higher medical expenses generally lead to larger settlements.
- Lost Wages and Earning Capacity: If the condition caused the plaintiff to miss work or reduced their ability to earn income, this financial loss will be considered in the settlement. Significant loss of income can result in larger compensation.
- Pain and Suffering: Non-economic damages such as physical pain, emotional distress, and loss of enjoyment of life are subjective but can substantially influence settlement amounts. Cases with significant pain and emotional trauma often see higher payouts.
- Legal and Expert Testimony: The strength of the evidence, including expert testimony linking Depo-Provera to the tumor, impacts the settlement. Strong, well-documented cases tend to achieve better outcomes.
- Punitive Damages: If the manufacturer (Pfizer) is found to have acted with gross negligence or willful misconduct, punitive damages may be awarded to punish and deter future misconduct, significantly increasing the payout.
- Settlement vs. Trial: Most cases are settled out of court, typically resulting in a guaranteed, though potentially lower, payout compared to what might be awarded by a jury at trial. A trial could result in a higher payout but also carries the risk of receiving nothing if the case is lost.
Settlements might range between $100,000 and $500,000 for smaller, treatable meningiomas with limited life impact. For large, debilitating tumors with extensive medical treatment and significant life impact, settlements or verdicts could range from $1 million to $5 million or more. Severe cases with solid evidence of negligence could also result in settlements or verdicts in the multi-million dollar range.
Settlement amounts vary widely and depend on each case’s specific details. Consulting with a leading attorney is essential to assessing the potential value of a Depo-Provera lawsuit and effectively navigating the legal process.
Depo-Provera Lawsuit Statute of Limitations and Deadlines
Statutes of limitations in Depo-Provera lawsuits vary by state and typically range from 1 to 6 years. The timeline potentially starts from the date of injury or discovery but may be extended by the discovery rule or if the person is a minor or incapacitated. Due to the significant variance in the statute of limitations for these kinds of cases, contacting an attorney is essential.
An attorney can help ensure timely filing and assist with collecting proper documentation. Affected individuals should start gathering necessary documentation early, as it can take time to collect medical records and proof of Depo-Provera use.
Depo-Provera lawsuits are generally based on personal injury or product liability claims. For personal injury claims, most states have a statute of limitations between 2 to 3 years from the date of injury or discovery. Product liability claims have similar time frames, with the clock starting when the injury or harm is discovered.
The statute of limitations may begin when the brain tumor or meningioma is diagnosed or when the condition is linked to Depo-Provera, extending the filing period if the injury wasn’t immediately apparent. It may be paused if the plaintiff is a minor or legally incapacitated, starting once they turn 18 or regain capacity.
Additionally, different time frames apply for wrongful death claims, usually starting from the date of death. An attorney with extensive experience in pharmaceutical litigation can determine the specific statute of limitations for your case and any applicable exceptions.
Contact a Depo-Provera Lawyer
Depo-Provera patients who are diagnosed with a brain tumor or meningioma are encouraged to contact King Law to schedule a free consultation. The attorneys at King Law are well-versed in handling pharmaceutical litigation and will work strategically to secure the compensation you deserve. Our legal team is committed to advocating for those affected by Depo-Provera, ensuring that each case is handled with expertise and care.

 Depo-Provera Brain Tumor Lawsuit: A Comprehensive Guide
Depo-Provera Brain Tumor Lawsuit: A Comprehensive Guide